In fusion reactions to make superheavy elements, energy is required to bring the two nuclei together and to form additional mass. How much energy must be converted into mass for the alpha bombardment of 253ES to form 257Md? (The isotopic masses are 253ES = 253.08294 amu, 4He = 4.00260 amu, 257Md = 257.09558 amu.) 3,01 x 106 kl/mol O 9,04 x 108 k/mol O 3.59 x 1011 k/mol wmol '0ו וכר O
In fusion reactions to make superheavy elements, energy is required to bring the two nuclei together and to form additional mass. How much energy must be converted into mass for the alpha bombardment of 253ES to form 257Md? (The isotopic masses are 253ES = 253.08294 amu, 4He = 4.00260 amu, 257Md = 257.09558 amu.) 3,01 x 106 kl/mol O 9,04 x 108 k/mol O 3.59 x 1011 k/mol wmol '0ו וכר O
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter18: Nuclear Reactions
Section: Chapter Questions
Problem 78QAP
Related questions
Question
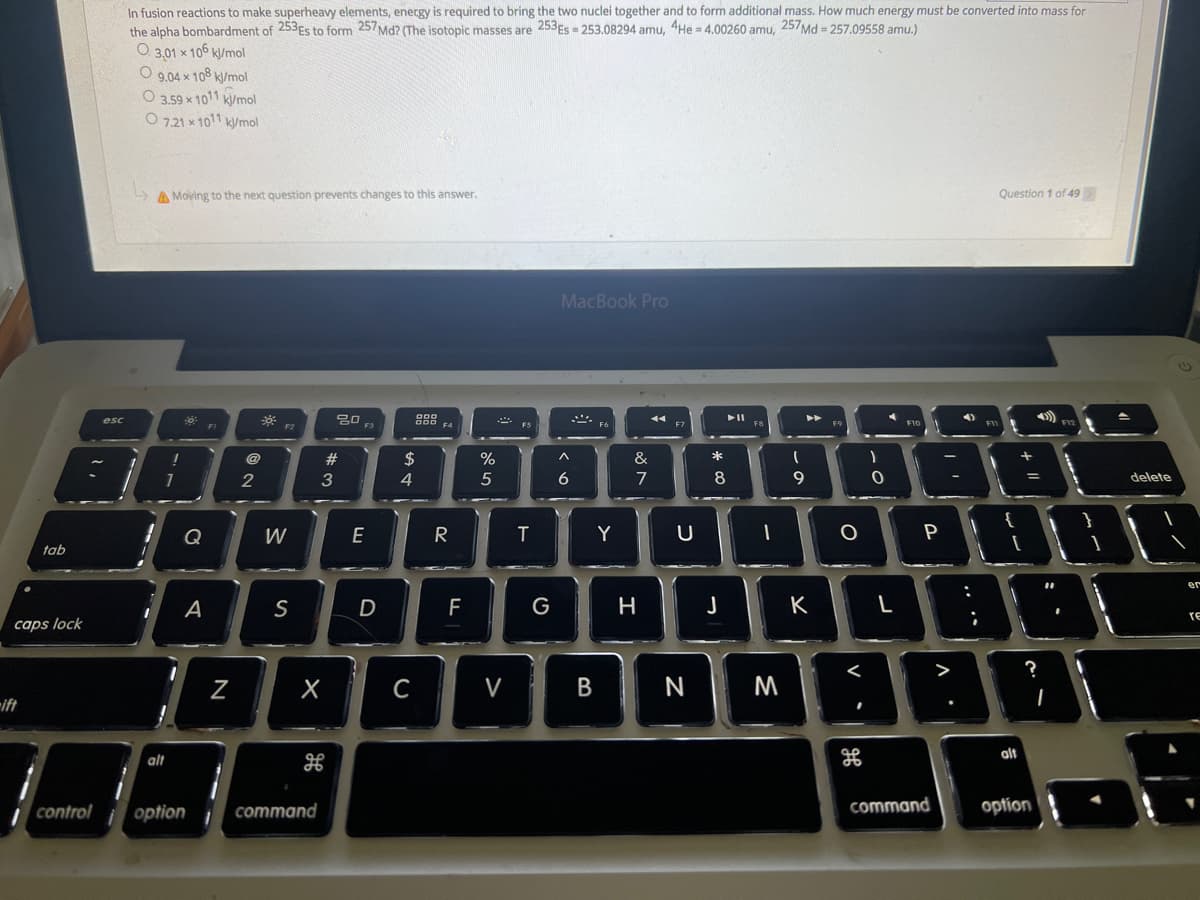
Transcribed Image Text:In fusion reactions to make superheavy elements, energy is required to bring the two nuclei together and to form additional mass. How much energy must be converted into mass for
the alpha bombardment of 253Es to form 257Md? (The isotopic masses are 253ES = 253.08294 amu, 4He = 4,00260 amu, 257Md = 257.09558 amu.)
O 3,01 x 106 k/mol
O 9,04 x 108 k/mol
O 3.59 x 1011 k/mol
mol ''0וא ו2ר O
%3D
%3D
A Moving to the next question prevents changes to this answer.
Question 1 of 49
MacBook Pro
esc
44
F2
F4
F5
F6
F7
F8
F10
F11
F12
F3
@
#3
$
&
*
2
3
6
7
8
9
delete
{
Q
W
E
R
T
Y
U
P
tab
er
S
D
G
H
K
re
caps lock
<
V
N
M
ift
olt
alt
control
option i
command
option
command
,. ..
* cO
B
N
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning