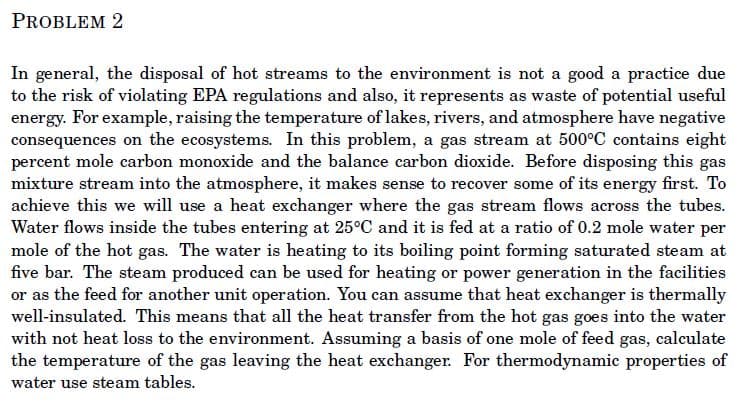
In general, the disposal of hot streams to the environment is not a good a practice due to the risk of violating EPA regulations and also, it represents as waste of potential useful energy. For example, raising the temperature of lakes, rivers, and atmosphere have negative consequences on the ecosystems. In this problem, a gas stream at 500°C contains eight percent mole carbon monoxide and the balance carbon dioxide. Before disposing this gas mixture stream into the atmosphere, it makes sense to recover some of its energy first. To achieve this we will use a heat exchanger where the gas stream flows across the tubes. Water flows inside the tubes entering at 25°C and it is fed at a ratio of 0.2 mole water per mole of the hot gas. The water is heating to its boiling point forming saturated steam at five bar. The steam produced can be used for heating or power generation in the facilities or as the feed for another unit operation. You can assume that heat exchanger is thermally well-insulated. This means that all the heat transfer from the hot gas goes into the water with not heat loss to the environment. Assuming a basis of one mole of feed gas, calculate the temperature of the gas leaving the heat exchanger. For thermodynamic properties of water use steam tables.
In general, the disposal of hot streams to the environment is not a good a practice due to the risk of violating EPA regulations and also, it represents as waste of potential useful energy. For example, raising the temperature of lakes, rivers, and atmosphere have negative consequences on the ecosystems. In this problem, a gas stream at 500°C contains eight percent mole carbon monoxide and the balance carbon dioxide. Before disposing this gas mixture stream into the atmosphere, it makes sense to recover some of its energy first. To achieve this we will use a heat exchanger where the gas stream flows across the tubes. Water flows inside the tubes entering at 25°C and it is fed at a ratio of 0.2 mole water per mole of the hot gas. The water is heating to its boiling point forming saturated steam at five bar. The steam produced can be used for heating or power generation in the facilities or as the feed for another unit operation. You can assume that heat exchanger is thermally well-insulated. This means that all the heat transfer from the hot gas goes into the water with not heat loss to the environment. Assuming a basis of one mole of feed gas, calculate the temperature of the gas leaving the heat exchanger. For thermodynamic properties of water use steam tables.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter9: Heat Transfer With Phase Change
Section: Chapter Questions
Problem 9.28P
Related questions
Concept explainers
Heat Exchangers
Heat exchangers are the types of equipment that are primarily employed to transfer the thermal energy from one fluid to another, provided that one of the fluids should be at a higher thermal energy content than the other fluid.
Heat Exchanger
The heat exchanger is a combination of two words ''Heat'' and ''Exchanger''. It is a mechanical device that is used to exchange heat energy between two fluids.
Question
Transcribed Image Text:PROBLEM 2
In general, the disposal of hot streams to the environment is not a good a practice due
to the risk of violating EPA regulations and also, it represents as waste of potential useful
energy. For example, raising the temperature of lakes, rivers, and atmosphere have negative
consequences on the ecosystems. In this problem, a gas stream at 500°C contains eight
percent mole carbon monoxide and the balance carbon dioxide. Before disposing this gas
mixture stream into the atmosphere, it makes sense to recover some of its energy first. To
achieve this we will use a heat exchanger where the gas stream flows across the tubes.
Water flows inside the tubes entering at 25°C and it is fed at a ratio of 0.2 mole water per
mole of the hot gas. The water is heating to its boiling point forming saturated steam at
five bar. The steam produced can be used for heating or power generation in the facilities
or as the feed for another unit operation. You can assume that heat exchanger is thermally
well-insulated. This means that all the heat transfer from the hot gas goes into the water
with not heat loss to the environment. Assuming a basis of one mole of feed gas, calculate
the temperature of the gas leaving the heat exchanger. For thermodynamic properties of
water use steam tables.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning