in kg of water. You cool the water to 40°C. How much K₂Cr₂O7 17. stayed dissolved in the 1 kg of water? 18 You prepared an exact saturated solution of K₂Cr₂O7 at 70°C in 1 kg of water. You cool the water to 40°C. How much K₂Cr₂O7 flocked out of solution as a solid? How much K₂Cr₂O7 must be dissolved into 1 kg of water at 90°C to make an exact saturated solution? 19
in kg of water. You cool the water to 40°C. How much K₂Cr₂O7 17. stayed dissolved in the 1 kg of water? 18 You prepared an exact saturated solution of K₂Cr₂O7 at 70°C in 1 kg of water. You cool the water to 40°C. How much K₂Cr₂O7 flocked out of solution as a solid? How much K₂Cr₂O7 must be dissolved into 1 kg of water at 90°C to make an exact saturated solution? 19
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 6QAP
Related questions
Question
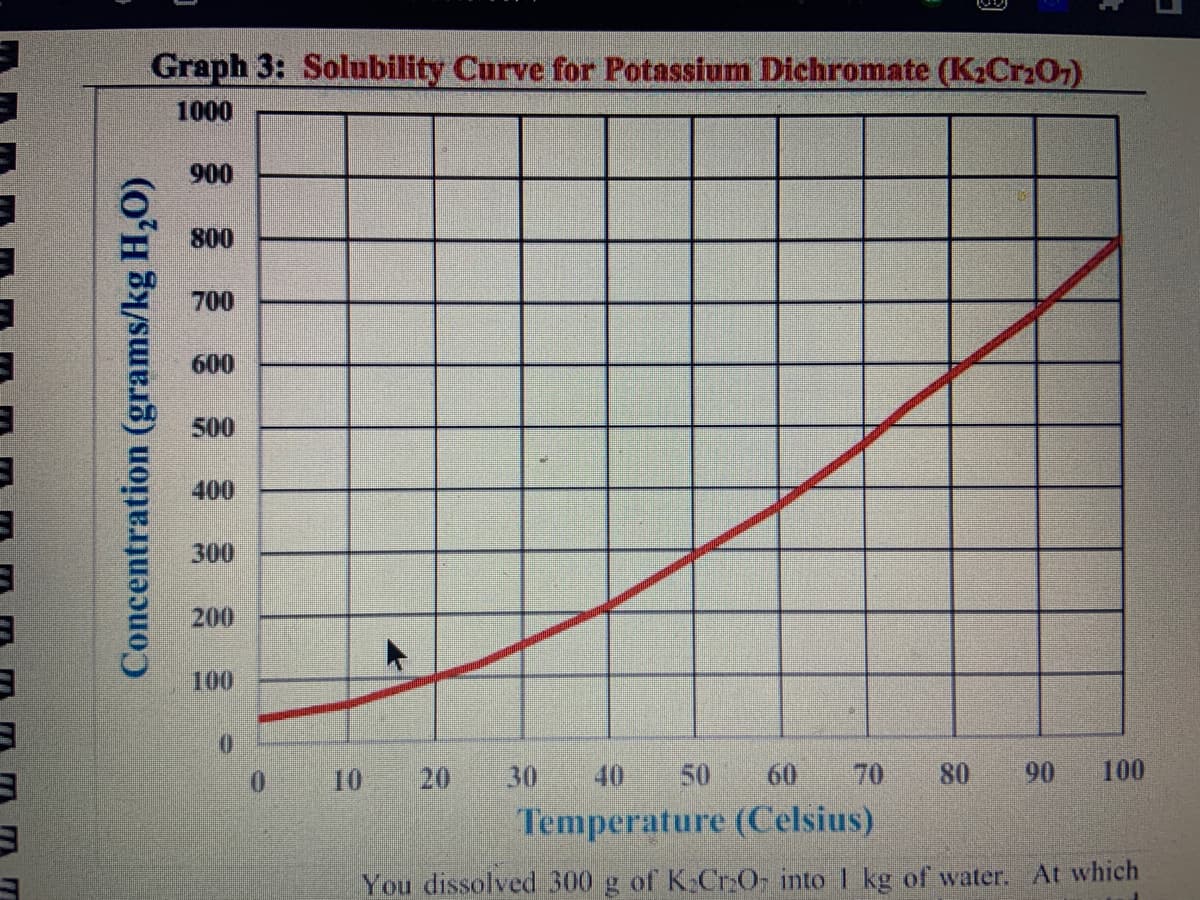
Transcribed Image Text:Graph 3: Solubility Curve for Potassium Dichromate (K₂Cr₂O7)
1000
Concentration (grams/kg H₂O)
900
800
700
600
500
400
300
200
100
0
10
►
10
60 70 80 90 100
Temperature (Celsius)
You dissolved 300 g of K₂Cr₂O into 1 kg of water. At which
20 30
1

Transcribed Image Text:0.01
You prepared an exact saturated solution of K₂Cr₂O7 at 70°℃ in 1
kg of water. You cool the water to 40°C. How much K₂Cr₂O7
17. stayed dissolved in the 1 kg of water?
18
19
20
21
You prepared an exact saturated solution of K₂Cr₂O7 at 70°C in 1
kg of water. You cool the water to 40°C. How much K₂Cr₂O7
flocked out of solution as a solid?
How much K₂Cr₂O7 must be dissolved into 1 kg of water at 90°C
to make an exact saturated solution?
You prepared an exact saturated solution of K₂Cr₂O7 at 90°℃ in 1
kg of water. You cool the water to 50°C. How much K₂Cr₂O7
stayed dissolved in the 1 kg of water?
You prepared an exact saturated solution of K₂Cr₂O7 at 90°C in 1
kg of water. You cool the water to 50°C. How much K₂Cr₂O₂
flocked out of solution as a solid?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning