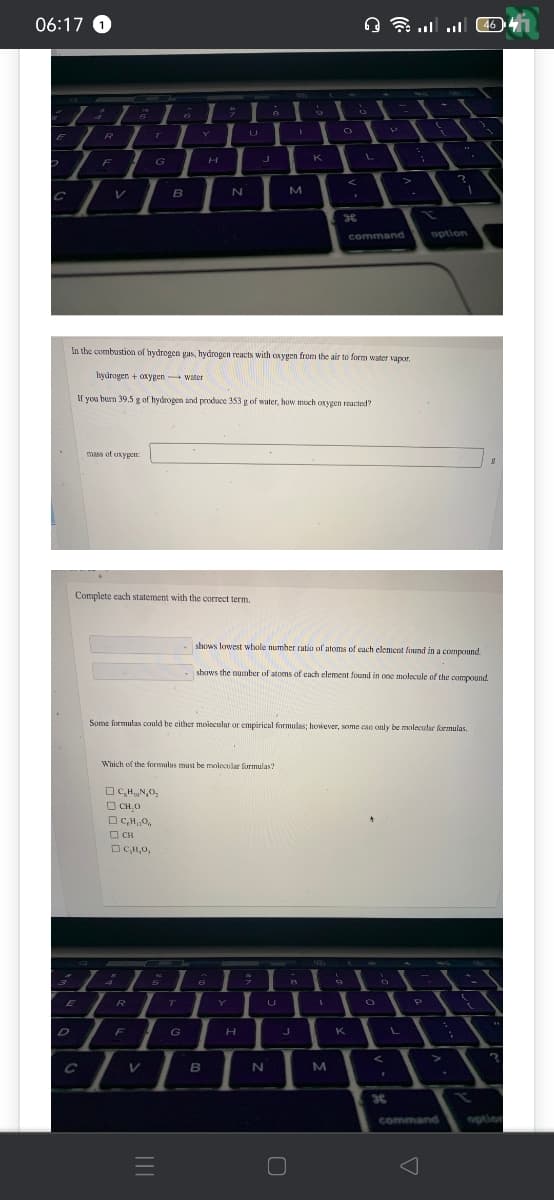
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen + oxygen-water If you burn 39.5 g of hydrogen and produce 353 g of water, how much oxygen reacted? mass of oxygen 8
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen + oxygen-water If you burn 39.5 g of hydrogen and produce 353 g of water, how much oxygen reacted? mass of oxygen 8
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter3: Chemical Bonds
Section: Chapter Questions
Problem 3.81P
Related questions
Question
Please send me the question in 20 minutes it's very urgent plz
Transcribed Image Text:06:17 1
E
D
C
4
E
D
R
F
V
mass of oxygen
24
T
R
G
C₂H₂O₂
□CH₂O
DC₂H₂O
CH
DC,1,0,
F
V
Complete each statement with the correct term.
B
N
5
||||
Y
=
Which of the formalas must be molecular formulas?
T
H
G
9
N
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor.
hydrogen + oxygen water
If you burn 39.5 g of hydrogen and produce 353 g of water, how much oxygen reacted?
6
U
B
J
8
H
| |
80
7
129
Some formulas could be either molecular or empirical formulas; however, some can only be molecular formulas.
N
I
M
U
K
B
1
M
F
(
shows lowest whole number ratio of atoms of each element found in a compound.
shows the number of atoms of each element found in one molecule of the compound.
9
9 - ... ...
L
K
1
command
1
P
O
O
TE
>
x
L
1
P
2
option
A
command
46¹41
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning