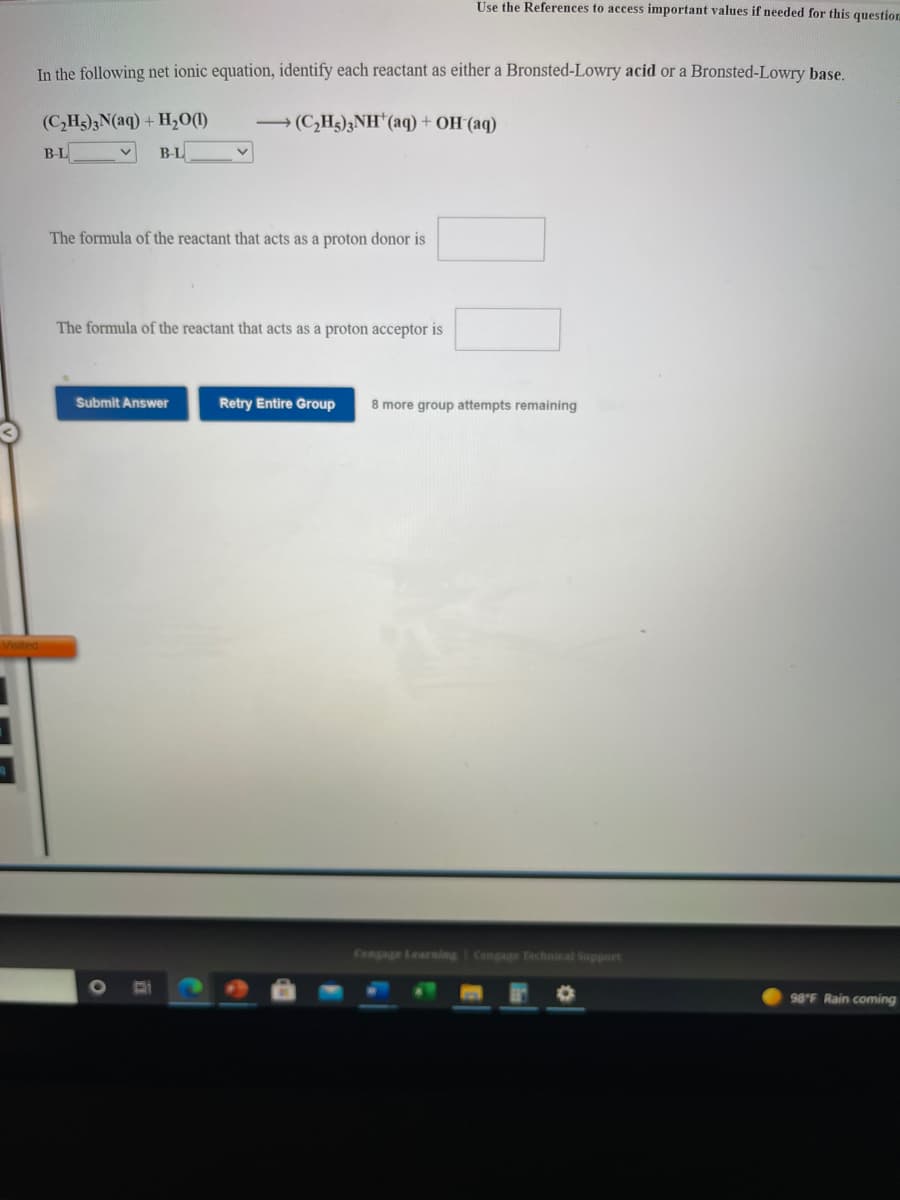
In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. (C,H)3N(aq) + H0(1) → (C,H3)3NH*(aq) + OH (aq) BL B-L The formula of the reactant that acts as a proton donor is The formula of the reactant that acts as a proton acceptor is Submit Answer Retry Entire Group 8 more group attempts remaining
In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base. (C,H)3N(aq) + H0(1) → (C,H3)3NH*(aq) + OH (aq) BL B-L The formula of the reactant that acts as a proton donor is The formula of the reactant that acts as a proton acceptor is Submit Answer Retry Entire Group 8 more group attempts remaining
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter17: Acid-base(proton Transfer) Reactions
Section: Chapter Questions
Problem 12E
Related questions
Question
Transcribed Image Text:Use the References to access important values if needed for this question
In the following net ionic equation, identify each reactant as either a Bronsted-Lowry acid or a Bronsted-Lowry base.
(C,H3)3N(aq) + H,0(1)
→ (C,H3)3NH*(aq) + OH(aq)
B-L
B-L
The formula of the reactant that acts as a proton donor is
The formula of the reactant that acts as a proton acceptor is
Submit Answer
Retry Entire Group
8 more group attempts remaining
Cengage Learning Cengage Technical Suppoet
98 F Rain coming
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning