In the gas phase, iodine (I2) reacts with cyclopentene (C5H8) by a free radical mechanism to produce cyclopentadiene (C5H6) and hydrogen iodide (HI) as shown in the given endothermic reaction. Describe how each of the following affects the amount of HI(g) present in the equilibrium mixture in the reaction. (A). When an inert gas such as He is added to a constant-volume reaction mixture, will the amount of HI(g) in equilibrium increase or decrease? (B). When the temperature of the reaction mixture is raised, will the amount of HI(g) in equilibrium increase or decrease? (C). When the volume of the container holding the mixture is doubled, will the amount of HI(g) in equilibrium increase or decrease?
In the gas phase, iodine (I2) reacts with cyclopentene (C5H8) by a free radical mechanism to produce cyclopentadiene (C5H6) and hydrogen iodide (HI) as shown in the given endothermic reaction. Describe how each of the following affects the amount of HI(g) present in the equilibrium mixture in the reaction.
(A). When an inert gas such as He is added to a constant-volume reaction mixture, will the amount of HI(g) in equilibrium increase or decrease?
(B). When the temperature of the reaction mixture is raised, will the amount of HI(g) in equilibrium increase or decrease?
(C). When the volume of the container holding the mixture is doubled, will the amount of HI(g) in equilibrium increase or decrease?
(D). When an appropriate catalyst is added to the reaction mixture, will the amount of HI(g) in equilibrium increase or decrease?
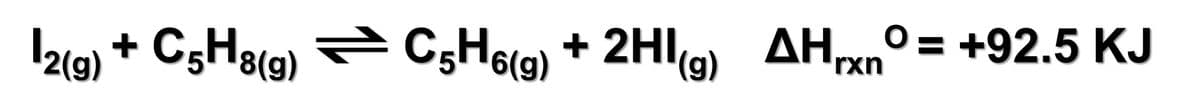
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images









