In the reaction of 10.00 mL of FeCl3(aq) and 12.4 g of K2C2O4(aq), you calculate that 9.58 g of K3FEC2O43H2O(s) will be produced. What does 9.58 g represent? percent yield limiting reactant actual yield molar mass
In the reaction of 10.00 mL of FeCl3(aq) and 12.4 g of K2C2O4(aq), you calculate that 9.58 g of K3FEC2O43H2O(s) will be produced. What does 9.58 g represent? percent yield limiting reactant actual yield molar mass
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter4: Recrystallization
Section: Chapter Questions
Problem 4Q
Related questions
Question
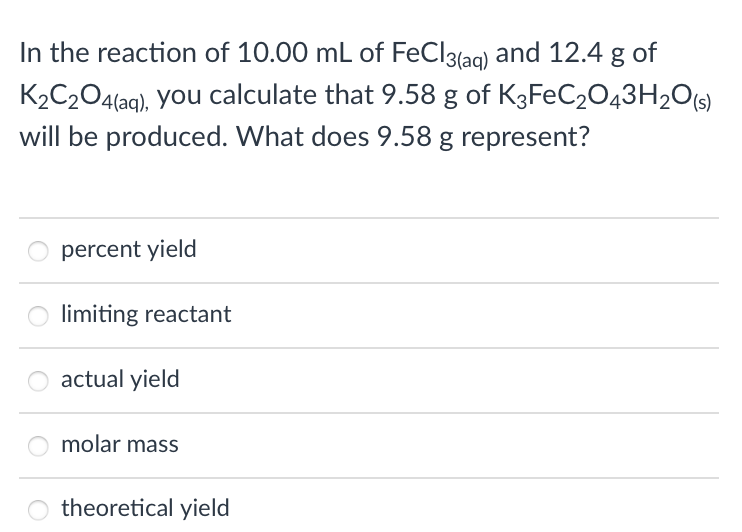
Transcribed Image Text:In the reaction of 10.00 mL of FeCl3{aq) and 12.4 g of
K2C204(aq), you calculate that 9.58 g of K3FEC2043H20[s)
will be produced. What does 9.58 g represent?
percent yield
limiting reactant
actual yield
molar mass
theoretical yield

Transcribed Image Text:Select all of the options below that are used to increase
the purity in the purification of crystals.
Stirring the solution after mixing.
Filtering the crystals
Recrystallization at least once.
Slow cooling during crystallization.
Heating the pure crystals
Fast cooling during crystallization.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning