In the smelting of iron ores, the reduction of iron oxides in a blast furnace is accompanied by the oxidation of CO(o) to CO2lg). The three reactions leading to the formation of Fe are: Reaction (i): 3 Fe,03(s) + CO(g) → 2 Fez04(s) + CO2(g) Reaction (ii): Fe304(s) + CO(g) → 3 FeO(s) + CO2(g) Reaction (iii): FeO(s) + CO(g) - Fe (s) + CO2(g) The graph below shows the variation of the Gibbs energy change with temperature for each of these reactions. Which one of the following statements is INCORRECT? 50 (iii) 40 -50 (ii) -100 -150 1000 2000 T/K O Reaction (iii) is spontaneous at high temperatures. O All of the three reactions are spontaneous at 500 K. O The Keg for Reaction (i) is larger than the Keg for Reaction (ii) at all temperatures. Reactions (i) and (ii) are more favorable at high temperatures. O O O O
In the smelting of iron ores, the reduction of iron oxides in a blast furnace is accompanied by the oxidation of CO(o) to CO2lg). The three reactions leading to the formation of Fe are: Reaction (i): 3 Fe,03(s) + CO(g) → 2 Fez04(s) + CO2(g) Reaction (ii): Fe304(s) + CO(g) → 3 FeO(s) + CO2(g) Reaction (iii): FeO(s) + CO(g) - Fe (s) + CO2(g) The graph below shows the variation of the Gibbs energy change with temperature for each of these reactions. Which one of the following statements is INCORRECT? 50 (iii) 40 -50 (ii) -100 -150 1000 2000 T/K O Reaction (iii) is spontaneous at high temperatures. O All of the three reactions are spontaneous at 500 K. O The Keg for Reaction (i) is larger than the Keg for Reaction (ii) at all temperatures. Reactions (i) and (ii) are more favorable at high temperatures. O O O O
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter21: Chemistry Of The Main-group Elements
Section: Chapter Questions
Problem 21.200QP
Related questions
Question
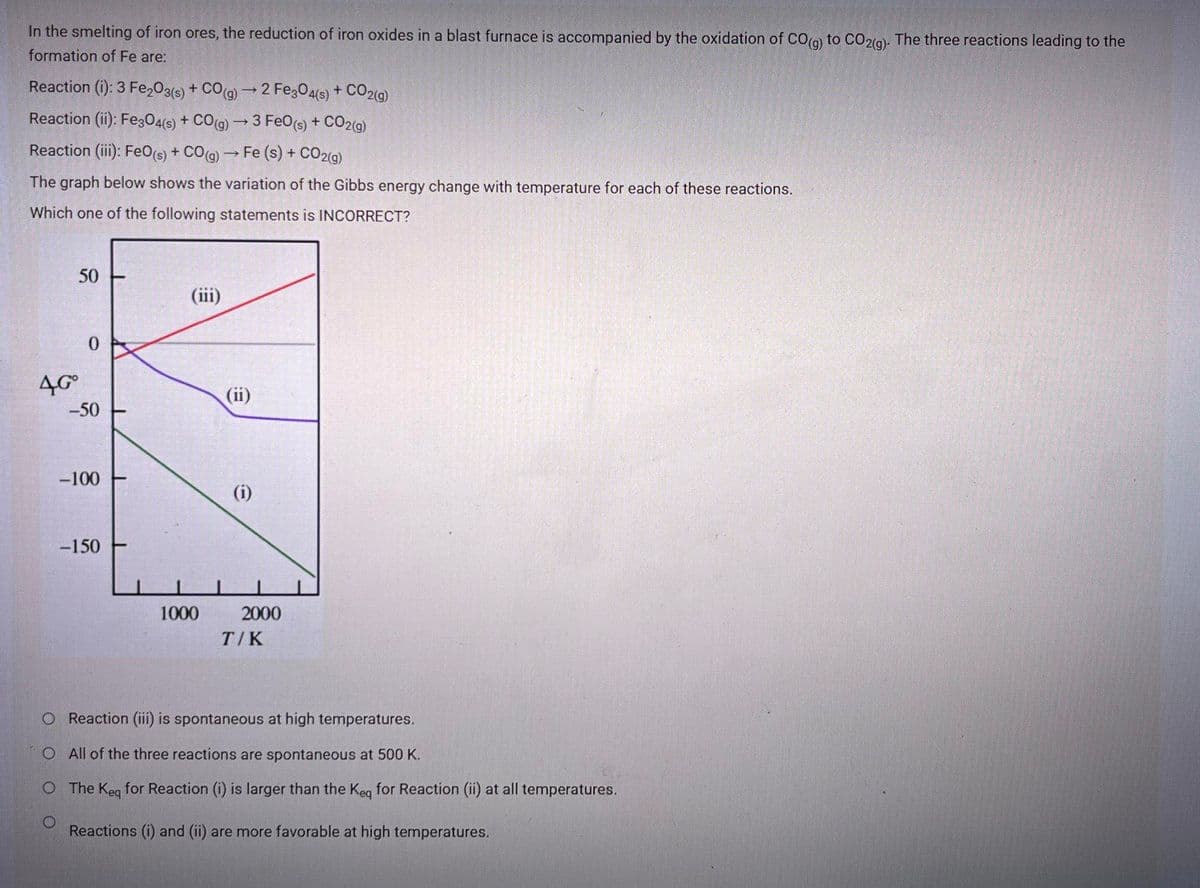
Transcribed Image Text:In the smelting of iron ores, the reduction of iron oxides in a blast furnace is accompanied by the oxidation of CO(o) to CO2(9). The three reactions leading to the
formation of Fe are:
Reaction (i): 3 Fe,03(s) + CO(g) → 2 FezO4(s) + CO2(g)
Reaction (ii): Fe304(s) + CO(9) 3 FeO(s) + CO29)
Reaction (ii): FeO(s) + CO(a) → Fe (s) + CO2(9)
The graph below shows the variation of the Gibbs energy change with temperature for each of these reactions.
Which one of the following statements is INCORRECT?
50
(ii)
4G°
(ii)
-50
-100
(i)
-150
1000
2000
T/K
O Reaction (iii) is spontaneous at high temperatures.
O All of the three reactions are spontaneous at 500 K.
O The Keg for Reaction (i) is larger than the Keg for Reaction (ii) at all temperatures.
Reactions (i) and (ii) are more favorable at high temperatures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
