In which of the following pure compounds would intermolecular H-bonding interactions be expected to have an effect on boiling point? Choose all that apply. O CH;CH2CH,OH CH3 CH3CHOCH,CH3 O CH3CH3 CH3 CH;CHCH2SH O None of the Above
In which of the following pure compounds would intermolecular H-bonding interactions be expected to have an effect on boiling point? Choose all that apply. O CH;CH2CH,OH CH3 CH3CHOCH,CH3 O CH3CH3 CH3 CH;CHCH2SH O None of the Above
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.40PAE: 8.40 Which of the following compounds would be expected to form intermolecular hydrogen bonds in the...
Related questions
Question
answer them please
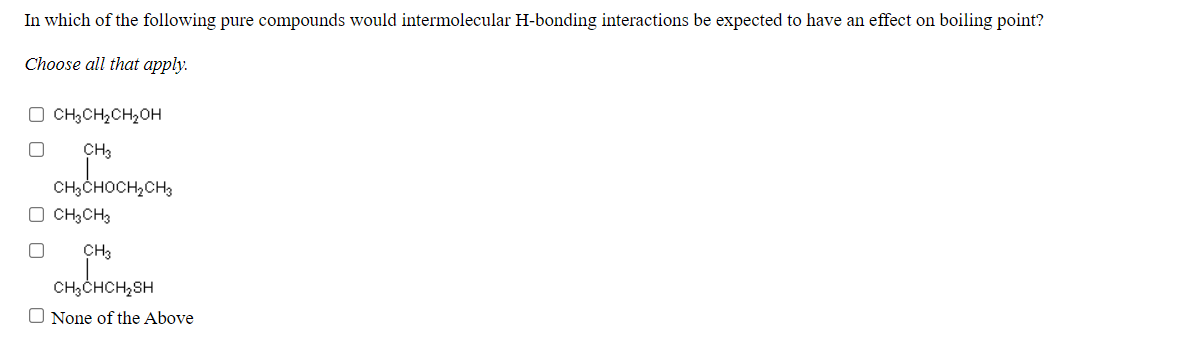
Transcribed Image Text:In which of the following pure compounds would intermolecular H-bonding interactions be expected to have an effect on boiling point?
Choose all that apply.
O CH;CH,CH2OH
CH3
CH3CHOCH,CH3
O CH3CH3
CH3
CH3CHCH,SH
O None of the Above
O O
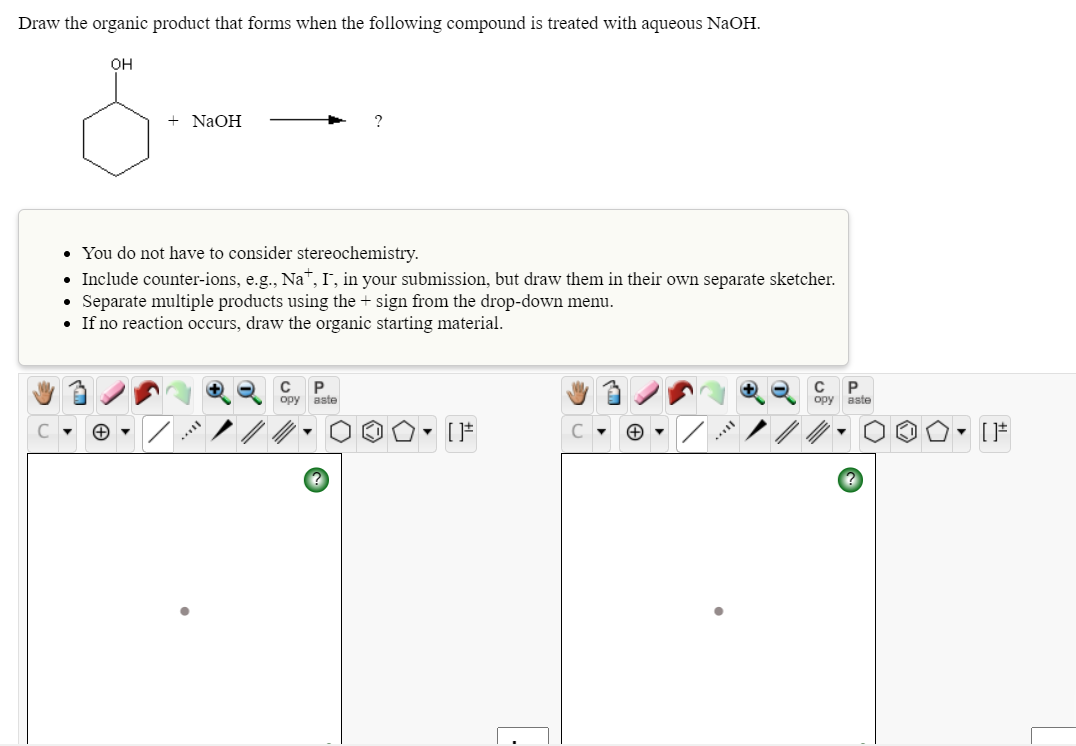
Transcribed Image Text:Draw the organic product that forms when the following compound is treated with aqueous NaOH.
он
+ NaOH
• You do not have to consider stereochemistry.
• Include counter-ions, e.g., Na™, I', in your submission, but draw them in their own separate sketcher.
Separate multiple products using the + sign from the drop-down menu.
• If no reaction occurs, draw the organic starting material.
ору
aste
opy aste
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax