In which of the following reactions will Kc = Kp? 2 SO3(s) + 2 NO(g) =2 SO2(3) + 2 NO2(3) 4 NH3(3) + 3 O2(g) = 2 N2(g) + 6 H2O(g) O 4 N2(8) + 2 O2(g) =4 N20(g) 6 SO2(s) + 3 O2lg) = 6 SO3(g)
Q: Calculate the value of the equilibrium constant, Ke, for the reaction Q(g) + X(g) = 2 M(g) + N(g)…
A: The equilibrium expression written for a reaction written in the reverse direction is the reciprocal…
Q: The equilibrium constant KP for the reaction 2SO3(g) ⇌ 2SO2(g) + O2(g) is 5.0 x 10-4 at 302°C.…
A: The equilibrium reaction is, 2SO3(g) ⇌ 2SO2(g) + O2(g) Given that, KP = 5.0 x 10-4 T = 302 °C =…
Q: Write the expressions for the equilibrium constants KP of these thermal decompositions: 2NaHCO3(s)…
A: Kp is equilibrium constant used when equilibrium concentrations are expressed in atmospheric…
Q: The equilibrium constant is given for two of the reactions below. Determine the value of the missing…
A: Reaction 1 Kc = K1 = 0.24 Reaction 2 Kc = K2 = 3.8 Reaction 3 Kc = K3 = ?
Q: Given that, at 700 K, Kp = 54.0 for the reaction H2(g) + I2(g) ↔ 2HI(g) and Kp = 1.04 x 10-4 for the…
A: Consider the reaction between H2 and I2. The Kp expression is as follows,
Q: For which of the following reactions is Kp = Kc? I. CO(g) + H20(1) = CO2(g) + H2(g) II. H2(g) +…
A: Given are five reactions.
Q: In which of the following equilibrium reactions will Kc be greater than Kp? O CH4(g) + 2O2(g) =…
A:
Q: Silver sulfate dissolves in water according to the reaction:…
A:
Q: Consider the following equilibr ium, for whichKp = 0.0752 at 480 °C:2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) +…
A:
Q: For the reaction NO(g) + ½ O2(g) ↔ NO2(g) at 750oC, the equilibrium constant Kc equal to…
A: The relationship between Kc and Kp is shown below,
Q: 6. The Kp for CH3NH2. 7. The Ka and Kb for dihydrogen phosphate.
A:
Q: 6) What is the relationship between Kp and Kc for the reaction: H2(g) + I2(@) 2Hl(9)
A:
Q: a particular temperature, the solubility of In₂(SO₄)₃ in water is 0.0060 M. (a) What is the…
A: KSP=?; S= 0.006=6*10-3M 1n2(SO4)3 ⩾2ln+3 + 3So4-2 2S-3 3S…
Q: Consider the reaction in the Haber process 3H2 (g) + N2 (g) → 2NH3 (g)where delta H° = -91.8 kJ/mol…
A: ΔH0 = -91.8 KJ/mol ΔS0 = -197 J/mol.K = -0.197 KJ/mol.K T= 25.0 oC = (25.0+273) K = 298 K
Q: The Kp for the following reaction is 0.036 at 385 ºC, what is Kc? PCl5(g)PCl3(g)+Cl2(g)
A: Answer
Q: For which of the following equilibria would the value of Kc equal the value of Kp? Select one: a.…
A:
Q: Calculate Kp for the following reaction: CH3OH (gas) =co1g) + Helg) 2 The following problem has an…
A:
Q: Analyses of an equilibrium mixture of N2O4(g) and NO2(g) gave the following results: [NO2(g)] =…
A: The equilibrium constant Kc for the following reaction can be expressed as follows-
Q: Which reactions shows where Kp is equal to Kc. 4 HCl(g) + O2(g) ⇌ 2 Cl2(g) + 2 H2O(g) 2 BrCl(g) ⇌…
A:
Q: Construct the expression for Kp for the following reaction. N:(g) + O:(g) = 2 NO(g)
A: Given :- N2(g) + O2(g) <----> 2NO(g) To write :- Kp expression for given equilibrium…
Q: How many of the following reactions will Kc = Kp? • H2(g) + I2(g) = 2 HI(g) • N204(g) = 2NO2(g) •…
A: The relation between the KC (equilibrium constant in the term of concentration) and KP (equilibrium…
Q: A mixture of CH4 and H2O is passed over a nickel catalystat 1000 K. The emerging gas is collected in…
A: A mixture of CH4 and H2O is passed over a nickel catalystat 1000 K The balanced chemical equation…
Q: Write the equations for the conversion of Kc to KP for each of the following reactions, which occur…
A: "since you have asked multiple questions in one question only first three will be answered."…
Q: In which of the following reactions will Ke = Kp? %3! O 4 NH3(g) +3 O2(g) 2 N2(g) + 6 H20(g) O 2…
A:
Q: 1. At 500°C, the reaction between N2 and H2 to form ammonia has Ke = 6.0 × 10-2. What is the %3D Kp…
A: Kp can be calculated as:-
Q: What is the Kp expression for the following reaction? 3Fe (s) + 4H2O (g) ⇌ Fe3O4 (s) + 4H2 (g)
A:
Q: there is an enzyme is catalyzed reaction that is at equilibrium and it produces 0.1 M of substrate…
A:
Q: What is the value of the equilibrium constant Kc for the following reaction at the temperature of…
A: Given [ NO2 ] = 4.313 × 10-3 M [ N2O4 ] = 2.706 × 10-3 M Equilibrium Constant ( Kc ) = ?
Q: or which of the following reactions is Kp = Kc (RT)? (Choose one option)
A: Equilibrium constant expressions Kp and Kc Kp and Kc relation : Kp = Kc (RT)∆n where…
Q: In which of the following reactions will Kp = Kc? %3D 6 S2 (3) O 4 P2 () + 12 H2S (3) = 8 PH3 () + 2…
A:
Q: Given the equilibrium constant values N2(g)+1/2O2(g)⇌N2O(g) KC=2.7×10−18 N2O4(g)⇌2NO2(g)…
A: The reactions given are 1) N2 (g) + 0.5 O2 (g) ⇌ N2O (g)…
Q: 3 In which of the following reactions will Kp = Kc? * 3A (g) + 2B(g) = 4E(g) + 6D(g) O A(s) +2B(g) =…
A: The relationship between Kp and Kc: Kp = Kc(RT)∆n Where, Kp = Equilibrium constant in terms of…
Q: The reaction below has a Kp value of 3.3 × 10-5. What is the value of Kc for this reaction at 700 K?…
A: Option E should be the correct answer (1.9x10-3) As per the given reaction we…
Q: For which of the following reactions are the numerical values of Kp and Kc the same.15 PCI:(g) =…
A: The relationship between Kp and Kc is,
Q: At 1000 K, Kp = 1.85 for the reactionSO2(g) + 1/2O2(g) ⇌ SO3(g)(a) What is the value of Kp for the…
A: a) Since the given reaction is SO2(g) + 1/2O2(g) ⇌ SO3(g) Kp = 1.85 = (PSO3) / {…
Q: the Kp for the following reaction is 0.029 at 379 C, what is Kc? PCl5(g) = PCl3(g)+Cl(g)
A: The following relation can be used to determine the value of kc: Kp = Kc(RT)Δng
Q: the reaction 2A(g)⇌B(g)+2C(g)2A(g)⇌B(g)+2C(g), a reaction vessel initially contains only A at a…
A: This problem can be solved by constructing the ICE table to find the equilibrium pressure of B and C…
Q: Consider the gas phase reaction: A(g) + 2 B(g) ⟺ C(g) Kp = 2.0 x 108 A reactor is…
A: Partial pressure of gas is the amount of pressure exerted by that individual gas in a mixture of…
Q: An important industrial process for synthesizing the ammonia used in agricultural fertilizers…
A: The relationship between Kp and Kc is given below.
Q: Barium sulphate is a relatively insoluble salt used for medical radiographs of the gastrointestinal…
A:
Q: 48). The concentration of magnesium carbonate in a saturated aqueous solution at 25°C is 1.87 × 10¯*…
A: Given sparingly soluble salt is magnesium carbonate. In a saturated aqueous solution, the…
Q: At 276.6 oC, the Keq for the reaction: 3A+5B ↔ 2AB3A+5B ↔ 2AB is 8.24⋅10−88.24⋅10-8 What is…
A: The given reaction is, 3A+5B ↔ 2AB Given that: temperature, T = 276.6 oC = (276.6 +273) K= 549.6 K…
Q: b. 2.55 x 10^-3
A:
Q: n the reaction CO(g) +2H2(g) ⇄ CH3OH(g) carried out at 483K, the following equilibrium…
A: The equilibrium constant which is expressed in terms of concentrations of the species involved is…
Q: The reaction below has Kc = 125 at 25ºC. What is the Kp value of the reaction? NH4HS (s) ↔️ NH3…
A: For the equilibrium: NH4HS (s) ↔️ NH3 (g) + H2S (g) Kc = 125 Temperature, T=25ºC= 25 + 273 = 298 K…
Q: Given that, at 700 K, Kp = 54.0 for the reactionH2(g) + I2(g) ⇌ 2 HI(g) and Kp = 1.04 x 10-4for the…
A: The given two reactions are,
Q: 4. Write Kp expressions for each of the following reactions: (a) C(s) + H₂O (g) H₂ (g) + CO (g) (b)…
A:
Q: For the following reaction, Kp= 137 at 3.50 * 102K. What is the value of Kc? 2C4H10 (l) + 3O2 (g)…
A: Given, Kp of the reaction is 137 Temperature of the reaction is 3.50 x 102 K = 350 K Formula Used,…
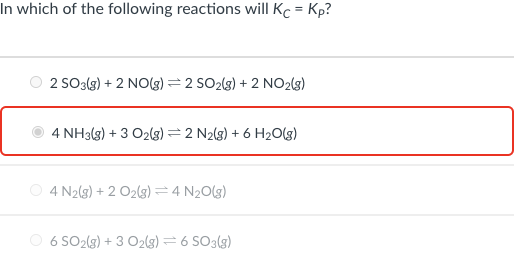
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Write the equations for the conversion of Kc to KP for each of the following reactions, which occur in the gas phase: (a) 2SO2(g) + O2(g) ⇌ 2SO3(g)(b) N2 O4(g) ⇌ 2NO2(g)(c) C3 H8(g) + 5O2(g) ⇌ 3CO2(g) + 4H2 O(g)(d) At 227 °C, the following reaction has Kc = 0.0952: CH3 OH(g) ⇌ CO(g) + 2H2(g)What would be the value of KP at this temperature?For which of the following equilibria would the value of Kc equal the value of Kp? Select one: a. CO (g) + 3 H2 (g) ⇋⇋ CH4 (g) + H2O (g) b. CO (g) + H2O (g) ⇋⇋ CO2 (g) + H2 (g) c. CO (g) + 2 H2 (g) ⇋⇋ CH3OH (g) d. CO (g) + ½ O2 (g) ⇋⇋ CO2 (g) e. H2 (g) + O2 (g) ⇋⇋ 2 H2O (l)For which one of the following reactions does Kp equal Kc? A. 4 NH3(g) + 3 O2(g) ⇌ 2 N2(g) + 6 H2O(g) B. SO3(g) + NO(g) ⇌ SO2(g) + NO2(g) C. 2 N2(g) + O2(g) ⇌ 2 N2O(g) D. 2 SO2(g) + O2(g) ⇌ 2 SO3(g) E. HBr(g) ⇌ 1212 H2(g) + 1212 Br2(l)
- For which of the following reactions is Kp = Kc (RT)? (Choose one option) a. C2H4 (g) + 2 O2 (g) ⇌ 2CO2 (g) + 2H2O (liq) b. 2 NO (g) + O2 (g) ⇌ 2 NO2 (g) c. C (s) + H2O (g) ⇌ H2 (g) + CO (g) d. 1/2 NO2 (g) + 1/2 H2O (g) ⇌ HNO3 (g) + 1/2 NO (g) e. NO (g) + O3 (g) ⇌ NO2 (g) + O2 (g)At 276.7 oC, the Kp for the reaction 2 A (g) + 1 B (g) ↔ 5 AB is Kp = 3.07⋅10-2 What is Keq?the Kp for the following reaction is 0.029 at 379 C, what is Kc? PCl5(g) = PCl3(g)+Cl(g)
- Calculate the KC for the reaction N2 (g) + 3H2 (g) ⇌ 2NH3 (g) at 773.15 K if the KP = 1.5 x 10 - 5 at this temperature.For which of the following equations would the value of Kc = Kp? A) 2 NBr₃ (g) ⇌ N₂ (g) + 3 Br₂ (g) B) 2 NO₂ (g) ⇌ N₂O₄ (g) C) 2 A (g) + B (s) ⇌ 2 C (s) + D (g) D) 2 HI (g) ⇌ H₂ (g) + I₂ (g)At a particular temperature, the solubility of In₂(SO₄)₃ in water is 0.0060 M. (a) What is the value of Ksp? (b) If solid In₂(SO₄)₃ is added to a solution that already contains 0.200 M Na₂SO₄, what will the new solubility of the solid be? (c) If solid In₂(SO₄)₃ is added to a solution that already contains 0.150 M In(NO₃)₃, what will the new solubility of the solid be?
- Silver sulfate dissolves in water according to the reaction: Ag2SO4(s)⇌2Ag+(aq)+SO42−(aq)K=1.1×10−5Ag2SO4(s)⇌2Ag+(aq)+SO42−(aq)K=1.1×10−5 at 298 KKA 1.5-LL solution contains 6.55 gg of dissolved silver sulfate. Part A If additional solid silver sulfate is added to the solution, will it dissolve?At 276.6 oC, the Keq for the reaction: 3A+5B ↔ 2AB3A+5B ↔ 2AB is 8.24⋅10−88.24⋅10-8 What is Kp? Enter your answer in scientific notation.What is Δn for the following equation in relating Kc to Kp? 4 NH3(g) + 3 O2(g) ⇌ 2 N2(g) + 6 H2O(g) A) 3 B) -1 C) -2 D) 2 E) 1

