In your experiment, you select one piece of metal and heat it to 100.0 °C, and then select a second piece of metal and cool it to -10.0 °C. Both pieces of metal are then placed in the beaker of water and the temperatures equilibrated. You want to select two pieces of metal to use such that the temperature changes the least, that is, the final temperature should be as near to 21.0 °C as possible. What piece of metal will you heat (choose its number)? What piece of metal will you cool (choose its number)? What is the final temperature of the water? Final temperature = °℃
In your experiment, you select one piece of metal and heat it to 100.0 °C, and then select a second piece of metal and cool it to -10.0 °C. Both pieces of metal are then placed in the beaker of water and the temperatures equilibrated. You want to select two pieces of metal to use such that the temperature changes the least, that is, the final temperature should be as near to 21.0 °C as possible. What piece of metal will you heat (choose its number)? What piece of metal will you cool (choose its number)? What is the final temperature of the water? Final temperature = °℃
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter6: Forced Convection Over Exterior Surfaces
Section: Chapter Questions
Problem 6.25P
Related questions
Question
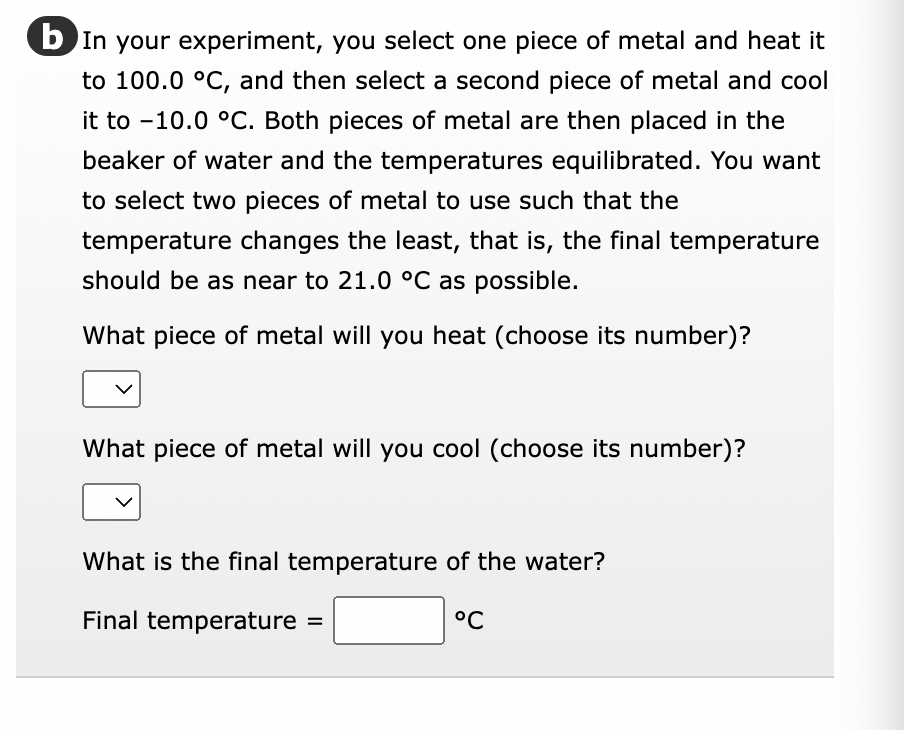
Transcribed Image Text:b In your experiment, you select one piece of metal and heat it
to 100.0 °C, and then select a second piece of metal and cool
it to -10.0 °C. Both pieces of metal are then placed in the
beaker of water and the temperatures equilibrated. You want
to select two pieces of metal to use such that the
temperature changes the least, that is, the final temperature
should be as near to 21.0 °C as possible.
What piece of metal will you heat (choose its number)?
What piece of metal will you cool (choose its number)?
What is the final temperature of the water?
Final temperature
°℃
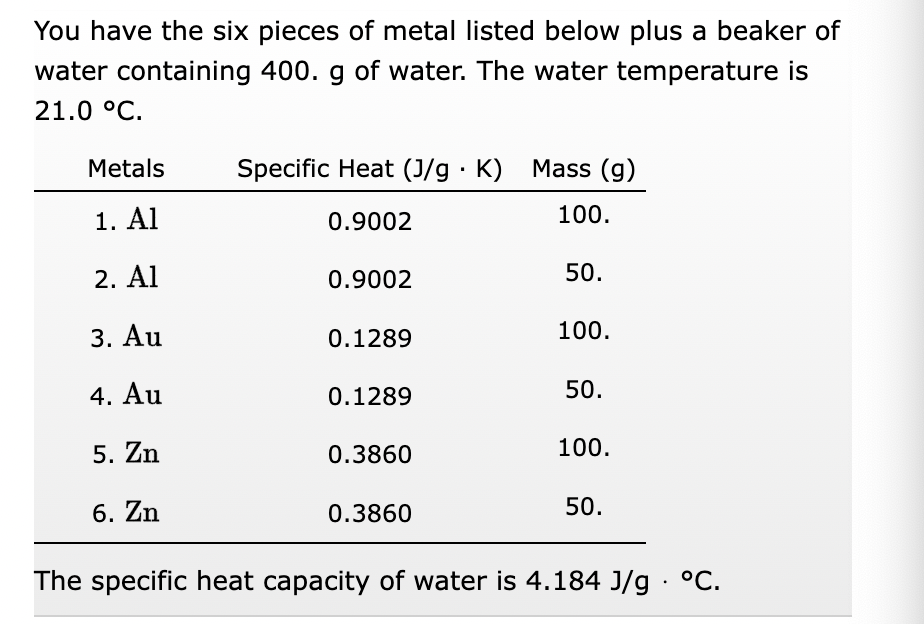
Transcribed Image Text:You have the six pieces of metal listed below plus a beaker of
water containing 400. g of water. The water temperature is
21.0 °C.
Metals Specific Heat (J/g. K) Mass (g)
1. Al
0.9002
100.
2. Al
3. Au
4. Au
5. Zn
6. Zn
0.9002
0.1289
0.1289
0.3860
0.3860
50.
100.
50.
100.
50.
The specific heat capacity of water is 4.184 J/g °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning