Iron ore is reduced to pure iron by smelting, during which the iron (III) oxide in the ore reacts with carbon monoxide gas, like this: Fe,0,() + 3C0 (g) → 2Fe(s)+3CO,(g) )+3CO (g) -> Suppose an engineer decides to study the rate of this reaction. He prepares four reaction vessels with 174.0 g of solid iron (III) oxide and 11.6 g of carbon monoxide gas each. The volume and temperature of each vessel is shown in the table below. Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. vessel volume temperature initial rate of reaction A 2.0 L 1100. °C 2.0 L 1200. °C ? 4.0 L 1100. °C 4.0 L 1000. °C
Iron ore is reduced to pure iron by smelting, during which the iron (III) oxide in the ore reacts with carbon monoxide gas, like this: Fe,0,() + 3C0 (g) → 2Fe(s)+3CO,(g) )+3CO (g) -> Suppose an engineer decides to study the rate of this reaction. He prepares four reaction vessels with 174.0 g of solid iron (III) oxide and 11.6 g of carbon monoxide gas each. The volume and temperature of each vessel is shown in the table below. Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. vessel volume temperature initial rate of reaction A 2.0 L 1100. °C 2.0 L 1200. °C ? 4.0 L 1100. °C 4.0 L 1000. °C
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 5ALQ: Consider the following statements: In general, the rate of a chemical reaction increases a bit at...
Related questions
Question
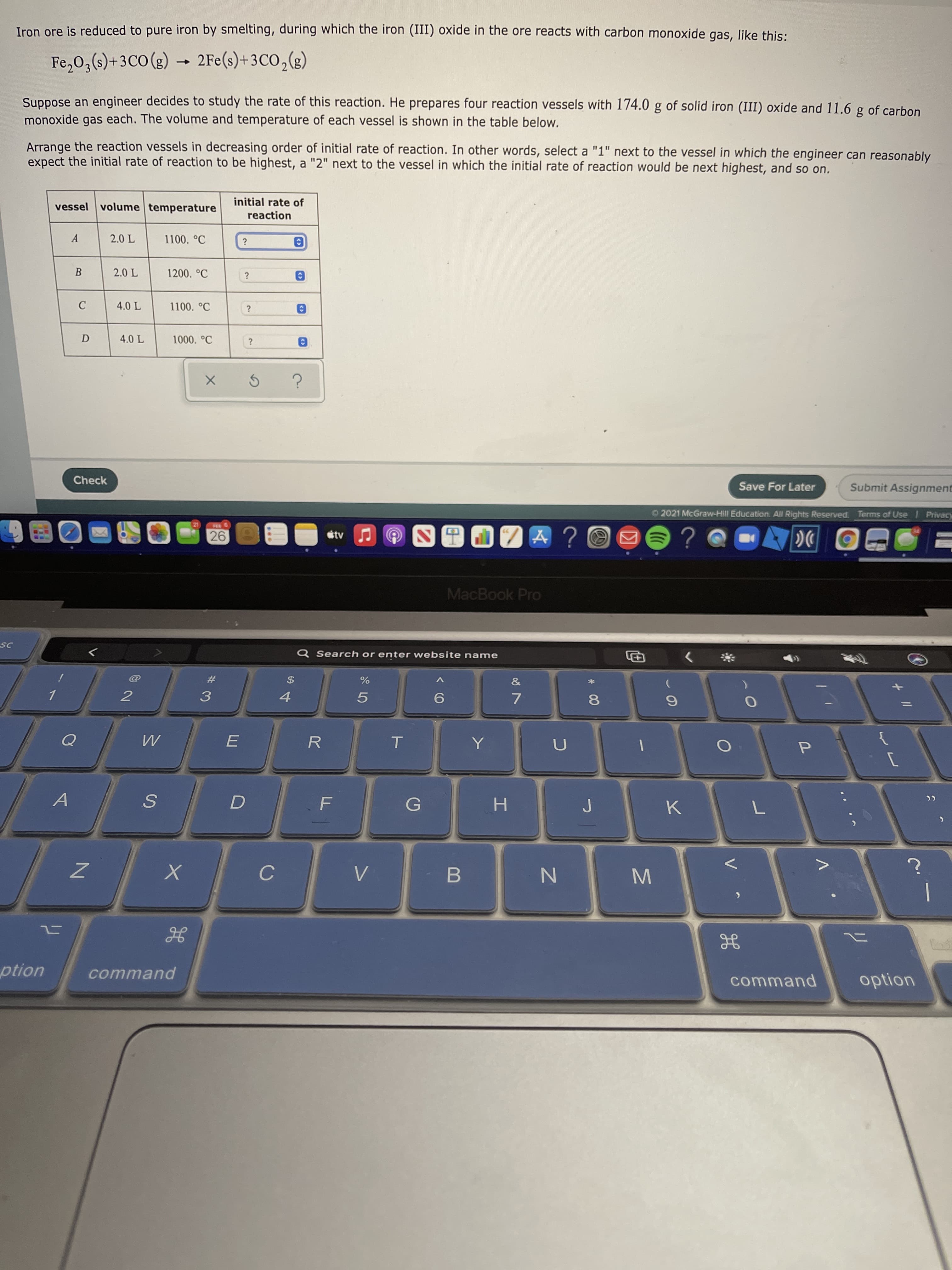
Transcribed Image Text:Iron ore is reduced to pure iron by smelting, during which the iron (III) oxide in the ore reacts with carbon monoxide gas, like this:
Fe,0,() + 3C0 (g) → 2Fe(s)+3CO,(g)
)+3CO (g)
->
Suppose an engineer decides to study the rate of this reaction. He prepares four reaction vessels with 174.0 g of solid iron (III) oxide and 11.6 g of carbon
monoxide gas each. The volume and temperature of each vessel is shown in the table below.
Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably
expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on.
vessel volume temperature
initial rate of
reaction
A
2.0 L
1100. °C
2.0 L
1200. °C
?
4.0 L
1100. °C
4.0 L
1000. °C
Expert Solution
Step 1
The above question involves the fundamentals of chemical kinetics.
The concentration terms are not used in this reaction.
The deciding factor therefore becomes the partial pressure.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning