Iron(III) chloride-thiocyanate system 1. Prepare a stock solution by adding 2 mL 0. 1 M FeCl̟ and 2 mL 0.1 M KSCN to 100 ml distilled water in a 250 – ml beaker. Note: The instructor will assign only one pair to prepare this stock solution for the whole class. 2. Prepare 4 test tubes (T1,T2,T3,T4). To each of the test tubes, add 2 mL of the stock solution. T1 will serve as the control. 3. To T2, add 10 drops 0. 1 M FeCl, To T3, add 10 drops 0. 1 M KSCN. To t4, add 3 drops 2.0 M NaOH. 4. Observe the colors of solutions and solids formed, if any. Test Tube 1 2 3 4 Observations (relative to control) Give the reaction equilibrium that describes this system:
Iron(III) chloride-thiocyanate system 1. Prepare a stock solution by adding 2 mL 0. 1 M FeCl̟ and 2 mL 0.1 M KSCN to 100 ml distilled water in a 250 – ml beaker. Note: The instructor will assign only one pair to prepare this stock solution for the whole class. 2. Prepare 4 test tubes (T1,T2,T3,T4). To each of the test tubes, add 2 mL of the stock solution. T1 will serve as the control. 3. To T2, add 10 drops 0. 1 M FeCl, To T3, add 10 drops 0. 1 M KSCN. To t4, add 3 drops 2.0 M NaOH. 4. Observe the colors of solutions and solids formed, if any. Test Tube 1 2 3 4 Observations (relative to control) Give the reaction equilibrium that describes this system:
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.10QAP
Related questions
Question
100%
[D] *refer to the photo below*
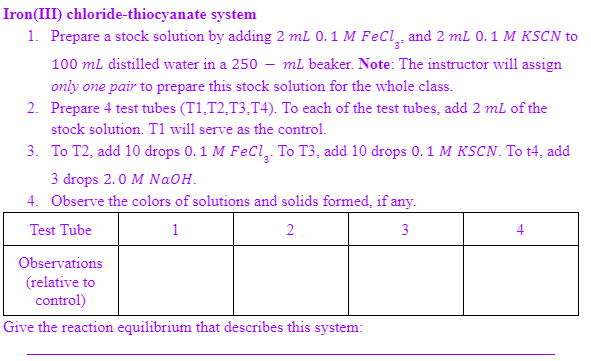
Transcribed Image Text:Iron(III) chloride-thiocyanate system
1. Prepare a stock solution by adding 2 mL 0.1 M FeCi̟ and 2 ml 0.1 M KSCN to
100 ml distilled water in a 250 – mL beaker. Note: The instructor will assign
only one pair to prepare this stock solution for the whole class.
2. Prepare 4 test tubes (T1,T2,T3,T4). To each of the test tubes, add 2 ml of the
stock solution. T1 will serve as the control.
3. To T2, add 10 drops 0.1 M FeCl, To T3, add 10 drops 0.1 M KSCN. To t4, add
3 drops 2.0 M NaOH.
4. Observe the colors of solutions and solids formed, if any.
Test Tube
1
2
3
4
Observations
(relative to
control)
Give the reaction equilibrium that describes this system:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

