It is often stated that the phosphate-phosphate bonds in ATP are "high energy," but in fact, they are not notably high in energy. Rather, they are easy to break, and the AG of hydrolysis is a "useful" quantity of energy. What makes the phosphate bonds easy to break? O They are close to the destabilizing nitrogenous base adenosine. O High alkalinity attacks bonds between phosphate groups. O Electrostatic repulsion of phosphate groups. O Positive charges on amino groups repel each other. High acidity attacks bonds between amino acids.
It is often stated that the phosphate-phosphate bonds in ATP are "high energy," but in fact, they are not notably high in energy. Rather, they are easy to break, and the AG of hydrolysis is a "useful" quantity of energy. What makes the phosphate bonds easy to break? O They are close to the destabilizing nitrogenous base adenosine. O High alkalinity attacks bonds between phosphate groups. O Electrostatic repulsion of phosphate groups. O Positive charges on amino groups repel each other. High acidity attacks bonds between amino acids.
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Lauralee Sherwood
Chapter2: Cell Physiology
Section: Chapter Questions
Problem 3SQE
Related questions
Question
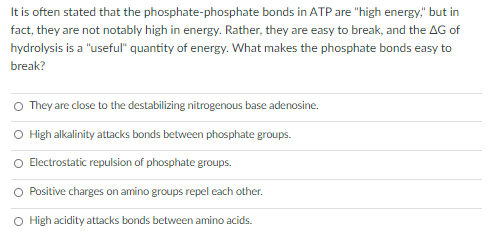
Transcribed Image Text:It is often stated that the phosphate-phosphate bonds in ATP are "high energy," but in
fact, they are not notably high in energy. Rather, they are easy to break, and the AG of
hydrolysis is a "useful" quantity of energy. What makes the phosphate bonds easy to
break?
O They are close to the destabilizing nitrogenous base adenosine.
O High alkalinity attacks bonds between phosphate groups.
O Electrostatic repulsion of phosphate groups.
Positive charges on amino groups repel each other.
O High acidity attacks bonds between amino acids.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Concepts of Biology
Biology
ISBN:
9781938168116
Author:
Samantha Fowler, Rebecca Roush, James Wise
Publisher:
OpenStax College

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Concepts of Biology
Biology
ISBN:
9781938168116
Author:
Samantha Fowler, Rebecca Roush, James Wise
Publisher:
OpenStax College