Javier mixes 20.00 mL of 0.500 M Pb(NO3)2 and 30.00 mL of 0.250 M NaCl in hopes of maki precipitate like all his other AP Chemistry friends! Did he do it? Did he make a precipitate? The Ksp of the possible precipitate is 1.6x10-S
Javier mixes 20.00 mL of 0.500 M Pb(NO3)2 and 30.00 mL of 0.250 M NaCl in hopes of maki precipitate like all his other AP Chemistry friends! Did he do it? Did he make a precipitate? The Ksp of the possible precipitate is 1.6x10-S
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 11E: The Handbook of Chemistry and Physics (http://openstaxcollege.org/l/16Handbook) gives solubilities...
Related questions
Question
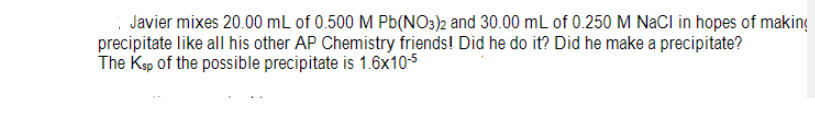
Transcribed Image Text:Javier mixes 20.00 mL of 0.500 M Pb(NO3)2 and 30.00 mL of 0.250 M NaCl in hopes of making
precipitate like all his other AP Chemistry friends! Did he do it? Did he make a precipitate?
The Ksp of the possible precipitate is 1.6x10-5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax