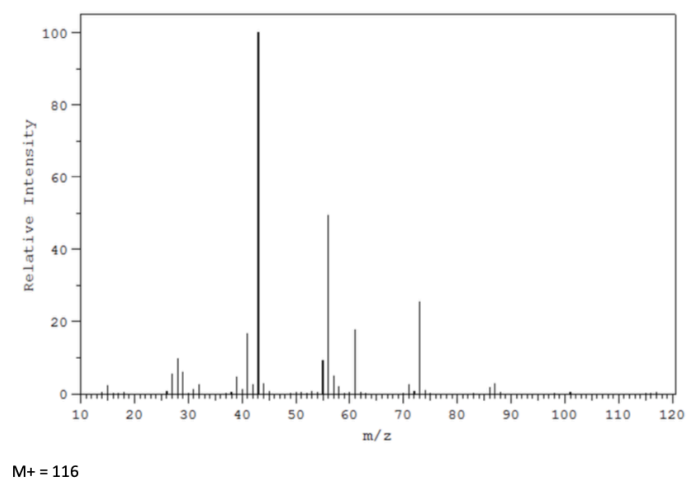
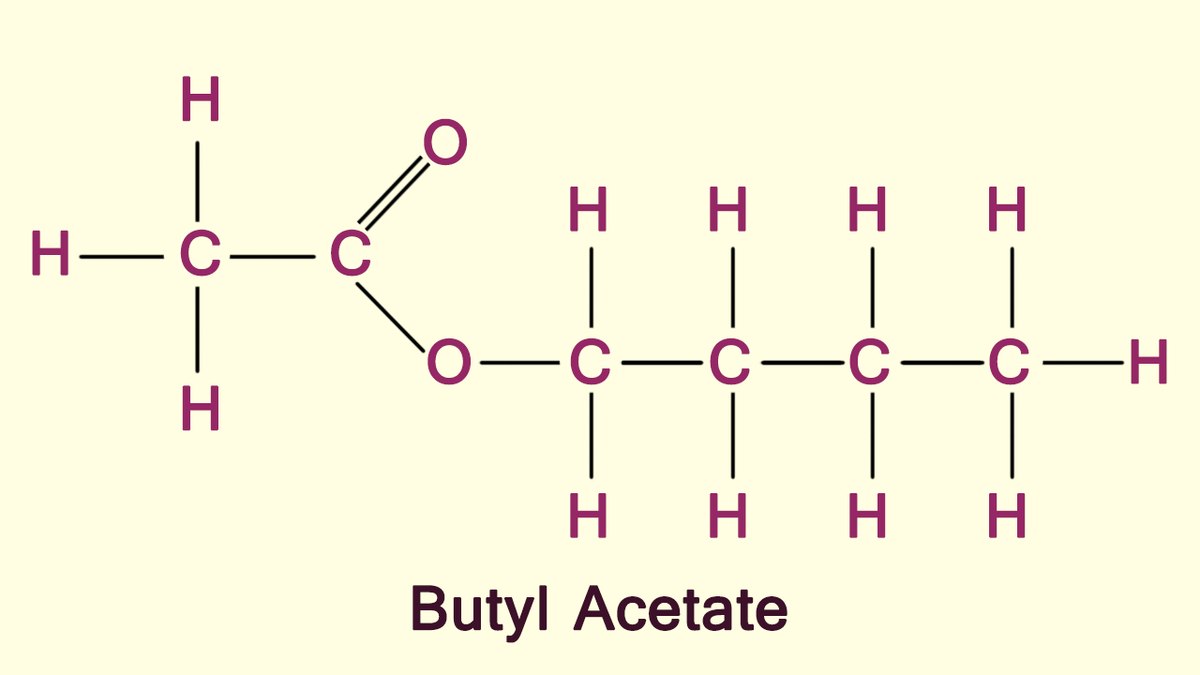
Can you help me break down the fragmentation of this mass spec spectra? Although the compound is not given, using the H NMR, C NMR and IR spectras that were also given, I found the compound to be CH3-COOH-CH2-CH2-CH2-CH3 (butyl acetate) In this Mass spec, it is given that M+=116 and I have found that fragmenting CH3 is 101, then CH2 is 87, and CH2 again is 73. I am just lost on where to go from there, while being left with CH3-COOH-CH2, because there are multiple peaks between 43 and 73 that I can't seem to figure out. Any help would be great!
Can you help me break down the fragmentation of this mass spec spectra? Although the compound is not given, using the H NMR, C NMR and IR spectras that were also given, I found the compound to be CH3-COOH-CH2-CH2-CH2-CH3 (butyl acetate) In this Mass spec, it is given that M+=116 and I have found that fragmenting CH3 is 101, then CH2 is 87, and CH2 again is 73. I am just lost on where to go from there, while being left with CH3-COOH-CH2, because there are multiple peaks between 43 and 73 that I can't seem to figure out. Any help would be great!
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.13QAP
Related questions
Question
Can you help me break down the fragmentation of this mass spec spectra? Although the compound is not given, using the H NMR, C NMR and IR spectras that were also given, I found the compound to be CH3-COOH-CH2-CH2-CH2-CH3 (butyl acetate)
In this Mass spec, it is given that M+=116 and I have found that fragmenting CH3 is 101, then CH2 is 87, and CH2 again is 73. I am just lost on where to go from there, while being left with CH3-COOH-CH2, because there are multiple peaks between 43 and 73 that I can't seem to figure out.
Any help would be great!
Transcribed Image Text:Relative Intensity
100
80
Ö
O
20
миниито
10
M+= 116
20
30 40
50
60
m/z
70
powqurubluqunuqtangguptag
100 110 120
80
90
Transcribed Image Text:H
H—C-C
Η
Η Η Η Η
-C-C-C-C-H
Η Η Η Η
Butyl Acetate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
