Ketones react with sodium acetylide (the sodium salt of acetylene, Na*-:C=CH) to give alcohols. For example, the reaction of sodium acetylide with 2-butanone yields 3-methyl-1-pentyn-3-ol: H3C OH 1. Na+-:C=CH H3C CH2CH3 2. H30+ CH2CH3 HC=C 2-Butanone 3-Methyl-1-pentyn-3-ol (a) Is the product chiral? (b) Assuming that the reaction takes place with equal likelihood from both Re and Si faces of the carbonyl group, is the product optically active? Explain.
Ketones react with sodium acetylide (the sodium salt of acetylene, Na*-:C=CH) to give alcohols. For example, the reaction of sodium acetylide with 2-butanone yields 3-methyl-1-pentyn-3-ol: H3C OH 1. Na+-:C=CH H3C CH2CH3 2. H30+ CH2CH3 HC=C 2-Butanone 3-Methyl-1-pentyn-3-ol (a) Is the product chiral? (b) Assuming that the reaction takes place with equal likelihood from both Re and Si faces of the carbonyl group, is the product optically active? Explain.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section: Chapter Questions
Problem 18.38P: A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted...
Related questions
Question
Please help me how to solve this problem in detail so i can understand it. Thank you very much.
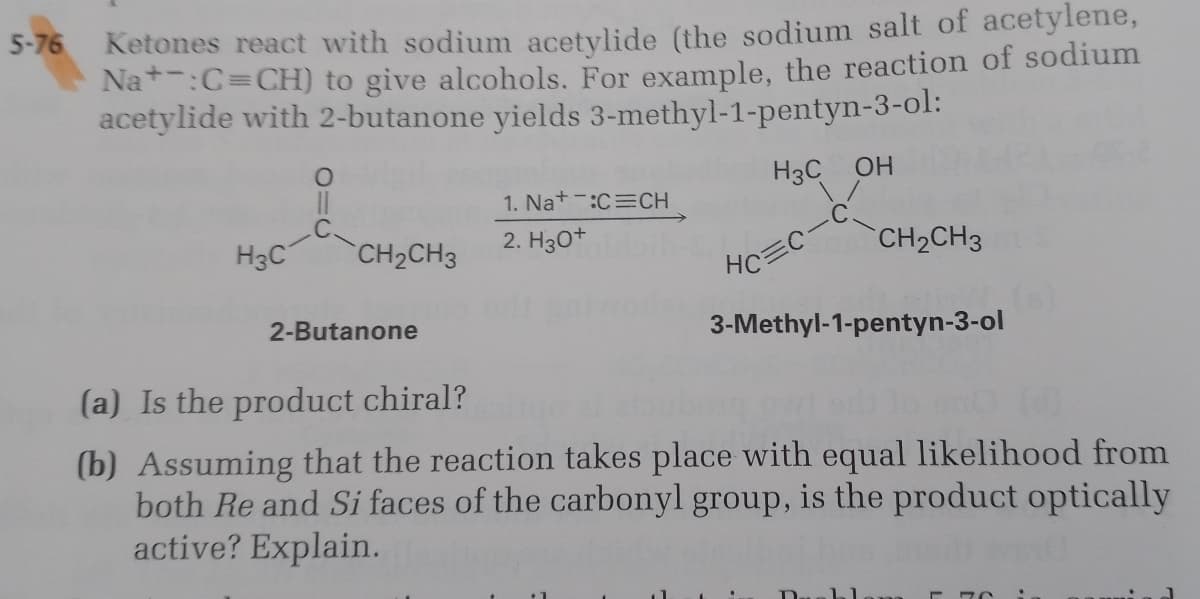
Transcribed Image Text:Ketones react with sodium acetylide (the sodium salt of acetylene,
Na*:C=CH) to give alcohols. For example, the reaction of sodium
acetylide with 2-butanone yields 3-methyl-1-pentyn-3-ol:
5-76
H3C OH
1. Nat-:C=CH
H3C
CH2CH3
2. Hзо+
CH2CH3
HC
2-Butanone
3-Methyl-1-pentyn-3-ol
(a) Is the product chiral?
(b) Assuming that the reaction takes place with equal likelihood from
both Re and Si faces of the carbonyl group, is the product optically
active? Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
