L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It can pick up a proton and become lactic acid(see right). The pKa of lactic acid is 3.86. A) Identify the ionizable oxygen that is partially protonated (50 % dissociated) at pH=3.86. B) Determine the acid dissociation constant, (Ka)?
L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It can pick up a proton and become lactic acid(see right). The pKa of lactic acid is 3.86. A) Identify the ionizable oxygen that is partially protonated (50 % dissociated) at pH=3.86. B) Determine the acid dissociation constant, (Ka)?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 116IL: Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO2H)...
Related questions
Question
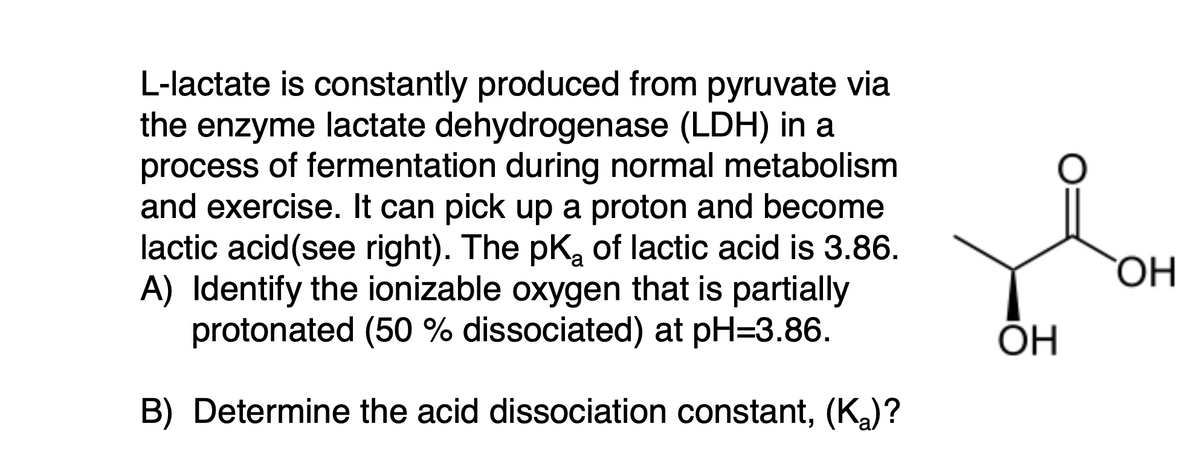
Transcribed Image Text:L-lactate is constantly produced from pyruvate via
the enzyme lactate dehydrogenase (LDH) in a
process of fermentation during normal metabolism
and exercise. It can pick up a proton and become
lactic acid(see right). The pK, of lactic acid is 3.86.
A) Identify the ionizable oxygen that is partially
protonated (50 % dissociated) at pH=3.86.
ÕH
B) Determine the acid dissociation constant, (Ka)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning