L.A certain gas occupies a space of 0.3 m3 at a pressure of 2 bar and a temperature of 77°C. It is heated at a constant volume, till the pressure is 7 bar. Determine (a) temperature at the end of the process (b) mass of the gas (c) change in internal energy (d) change in enthalpy. Assume cp = 1.005 kJ/kg K, cv = 0.712 kJ/kg K, R = 287 J/kg K.
L.A certain gas occupies a space of 0.3 m3 at a pressure of 2 bar and a temperature of 77°C. It is heated at a constant volume, till the pressure is 7 bar. Determine (a) temperature at the end of the process (b) mass of the gas (c) change in internal energy (d) change in enthalpy. Assume cp = 1.005 kJ/kg K, cv = 0.712 kJ/kg K, R = 287 J/kg K.
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 17RQ: At a constant pressure, how does a volume of gas vary with respect to the absolute temperature?
Related questions
Question
Q1
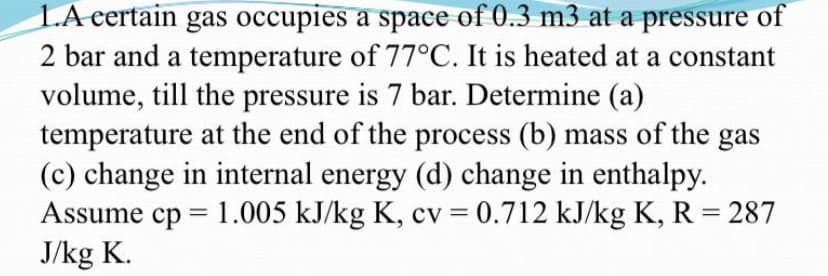
Transcribed Image Text:LA eertain gas occupies a space of 0.3 m3 at a pressure of
2 bar and a temperature of 77°C. It is heated at a constant
volume, till the pressure is 7 bar. Determine (a)
temperature at the end of the process (b) mass of the gas
(c) change in internal energy (d) change in enthalpy.
Assume cp = 1.005 kJ/kg K, cv = 0.712 kJ/kg K, R = 287
J/kg K.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning