la) Biliverdin is a pigment produced by tree frogs that causes them to look green - its structure in its fully ionized form is shown below. It contains two ionizable groups, both of which are carboxyl groups; one has a pK, of 3.9 and the other has a pK₂ of 5.3. What would the net charge on the whole molecule be if you dissolved it in an aqueous solution at pH 7.0? Briefly explain how you determined the charge. H₂C H₂C H3C Coo coo CH3 H3C H₂C 1b) What pH would you need to adjust the solution of biliverdin to in order to get this molecule to have a net charge of 0 (neutral or uncharged) essentially 100% of the time? Briefly explain why you chose that pH.
la) Biliverdin is a pigment produced by tree frogs that causes them to look green - its structure in its fully ionized form is shown below. It contains two ionizable groups, both of which are carboxyl groups; one has a pK, of 3.9 and the other has a pK₂ of 5.3. What would the net charge on the whole molecule be if you dissolved it in an aqueous solution at pH 7.0? Briefly explain how you determined the charge. H₂C H₂C H3C Coo coo CH3 H3C H₂C 1b) What pH would you need to adjust the solution of biliverdin to in order to get this molecule to have a net charge of 0 (neutral or uncharged) essentially 100% of the time? Briefly explain why you chose that pH.
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 7P: Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a...
Related questions
Question
can you please help with question 1b
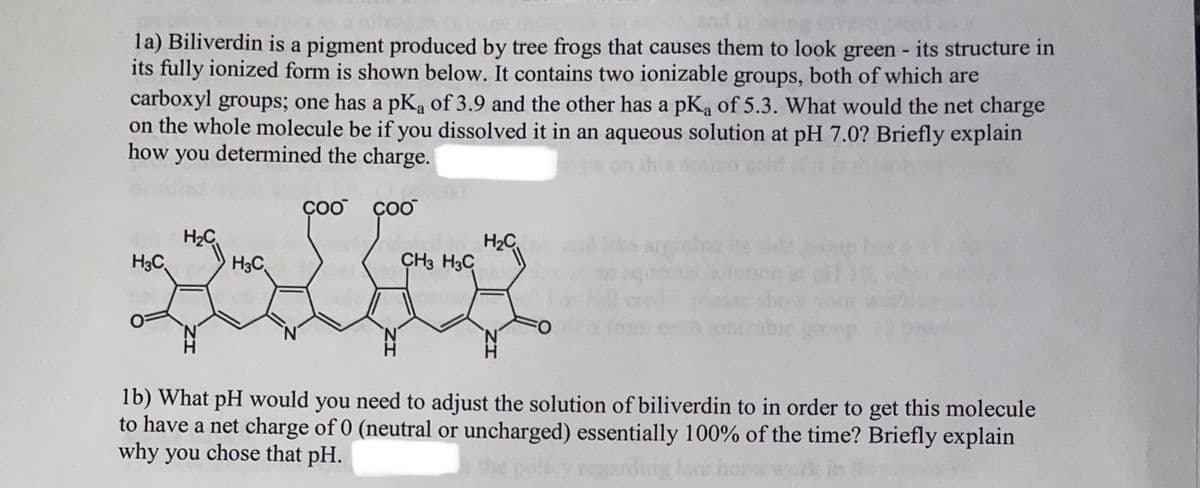
Transcribed Image Text:la) Biliverdin is a pigment produced by tree frogs that causes them to look green - its structure in
its fully ionized form is shown below. It contains two ionizable groups, both of which are
carboxyl groups; one has a pK₂ of 3.9 and the other has a pK₂ of 5.3. What would the net charge
on the whole molecule be if you dissolved it in an aqueous solution at pH 7.0? Briefly explain
how you determined the charge.
H3C
H₂C
N
H3C
COO COO
CH3 H3C
H₂C
FO
1b) What pH would you need to adjust the solution of biliverdin to in order to get this molecule
to have a net charge of 0 (neutral or uncharged) essentially 100% of the time? Briefly explain
why you chose that pH.
the policy regarding late
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning