le answer for each question and fill in the corresponding bubble on your Scantron form. 1. A solution contains 2.0 mol of CaS04. Which statement is true? A) The solution has -2.0 Eq of SO42". B) The solution has -4.0 Eq of SO42 C) The solution has 2.0 Eq of Ca2+. D) The solution has 4.0 Eq of Ca2+. 2. Which solution is the most acidic? A) 1.0 x 10-7M OH В) 2.3 х 10- М ОН С) 3.9 х 10-8 М ОН D) 5.1 x 10-2 M -OH E) 1.3 x 10-8 M -OH 3. Which substance is a nonelectrolyte? B) NaCl A) H2O2 С) КОН D) CASO4 1. Which compound will be the least soluble in water? A) H H H H-C-C -C-0–C-H H H H H. B) H HH H-0-Ç ---C-0-H H H.
le answer for each question and fill in the corresponding bubble on your Scantron form. 1. A solution contains 2.0 mol of CaS04. Which statement is true? A) The solution has -2.0 Eq of SO42". B) The solution has -4.0 Eq of SO42 C) The solution has 2.0 Eq of Ca2+. D) The solution has 4.0 Eq of Ca2+. 2. Which solution is the most acidic? A) 1.0 x 10-7M OH В) 2.3 х 10- М ОН С) 3.9 х 10-8 М ОН D) 5.1 x 10-2 M -OH E) 1.3 x 10-8 M -OH 3. Which substance is a nonelectrolyte? B) NaCl A) H2O2 С) КОН D) CASO4 1. Which compound will be the least soluble in water? A) H H H H-C-C -C-0–C-H H H H H. B) H HH H-0-Ç ---C-0-H H H.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 30QAP: A solution labeled “0.25 M AICl3” would contain _________ le(s) of Al3+ and mole(s) of Cl- in each...
Related questions
Question
#1
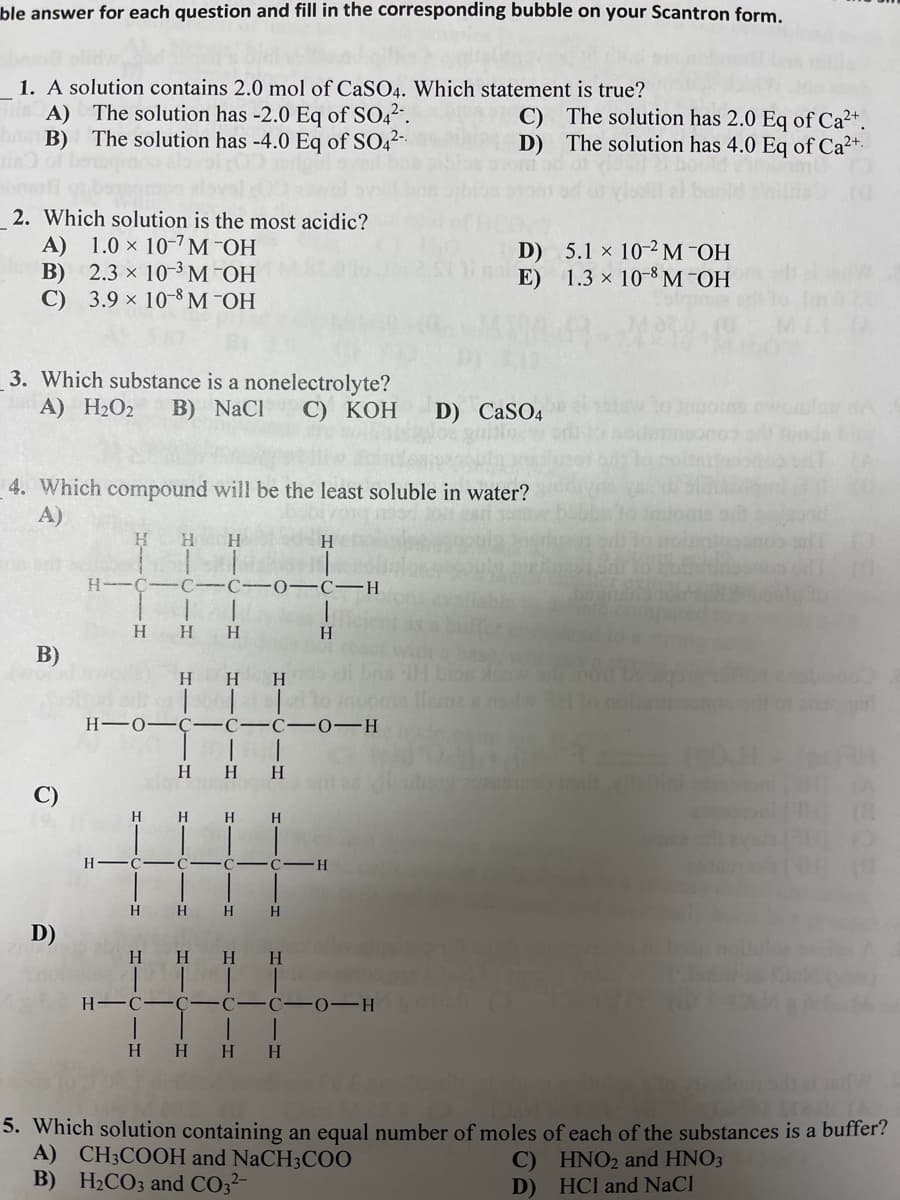
Transcribed Image Text:ble answer for each question and fill in the corresponding bubble on your Scantron form.
1. A solution contains 2.0 mol of CaSO4. Which statement is true?
A) The solution has -2.0 Eq of SO42.
The solution has -4.0 Eq of SO,2-
C) The solution has 2.0 Eq of Ca²+.
D) The solution has 4.0 Eq of Ca2+.
B)
2. Which solution is the most acidic?
A) 1.0 × 10-7M¯OH
В) 2.3 х 10-3 М ОН
C) 3.9 × 10-8 M¯OH
D) 5.1 x 10-2M¯OH
E)
1.3 × 10-8 M -OH
3. Which substance is a nonelectrolyte?
B) NaCl
A) H2O2
С) КОН
D) CaSO4
4. Which compound will be the least soluble in water?
A)
H.
H.
H
H.
C -C
C-H
H
H.
H.
H.
В)
H
H.
H-0–C-C-C -0-H
H
H
H.
H
H
H
H
H
H.
H.
H.
D)
H
H.
H.
H.
H -C
0-H
H H
H.
H.
5. Which solution containing an equal number of moles of each of the substances is a buffer?
A) CH3COOH and NaCH3C00
B) H2CO3 and CO32-
C) HNO2 and HNO3
D) HCl and NaCl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning