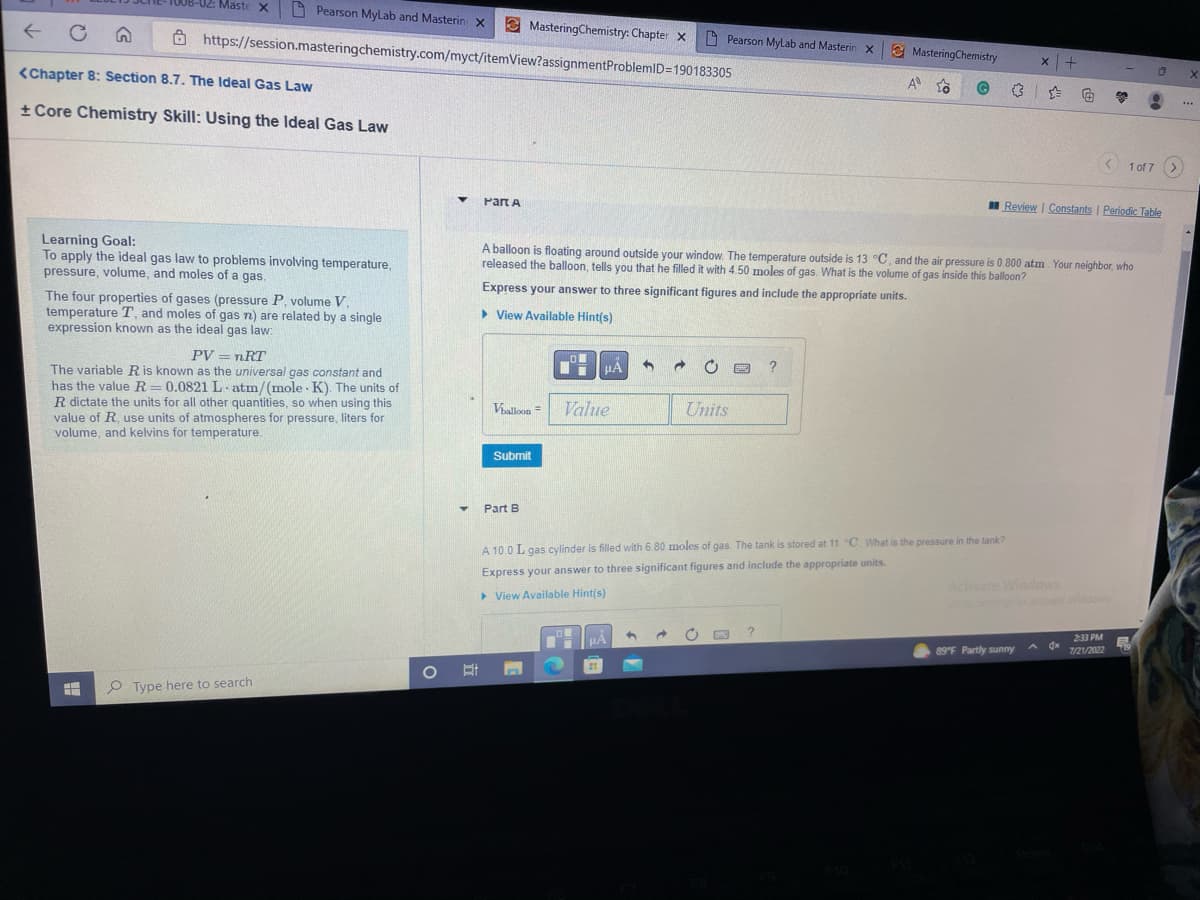
Learning Goal: To apply the ideal gas law to problems involving temperature, pressure, volume, and moles of a gas. The four properties of gases (pressure P. volume V. temperature T, and moles of gas n) are related by a single expression known as the ideal gas law: PV = nRT The variable R is known as the universal gas constant and has the value R=0.0821 L atm/(mole K). The units of R dictate the units for all other quantities, so when using this value of R, use units of atmospheres for pressure, liters for volume, and kelvins for temperature. A balloon is floating around outside your window. The temperature outside is 13 °C, and the air pressure is 0.800 atm. Your neighbor, who released the balloon, tells you that he filled it with 4.50 moles of gas. What is the volume of gas inside this balloon? Express your answer to three significant figures and include the appropriate units. ▸ View Available Hint(s) Vballoon = Submit Part B HOM Value 3 Units ? Constants | Periodic T A 10.0 L gas cylinder filled with 6.80 moles of gas. The tank is stored at 11 "C. What is the pressure in the tank? Express your answer to three significant figures and include the appropriate units. ▸ View Available Hint(s)
Learning Goal: To apply the ideal gas law to problems involving temperature, pressure, volume, and moles of a gas. The four properties of gases (pressure P. volume V. temperature T, and moles of gas n) are related by a single expression known as the ideal gas law: PV = nRT The variable R is known as the universal gas constant and has the value R=0.0821 L atm/(mole K). The units of R dictate the units for all other quantities, so when using this value of R, use units of atmospheres for pressure, liters for volume, and kelvins for temperature. A balloon is floating around outside your window. The temperature outside is 13 °C, and the air pressure is 0.800 atm. Your neighbor, who released the balloon, tells you that he filled it with 4.50 moles of gas. What is the volume of gas inside this balloon? Express your answer to three significant figures and include the appropriate units. ▸ View Available Hint(s) Vballoon = Submit Part B HOM Value 3 Units ? Constants | Periodic T A 10.0 L gas cylinder filled with 6.80 moles of gas. The tank is stored at 11 "C. What is the pressure in the tank? Express your answer to three significant figures and include the appropriate units. ▸ View Available Hint(s)
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
100%
Transcribed Image Text:Pearson MyLab and Masterin X
https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=190183305
-02: Maste X
<Chapter 8: Section 8.7. The Ideal Gas Law
+ Core Chemistry Skill: Using the Ideal Gas Law
Learning Goal:
To apply the ideal gas law to problems involving temperature,
pressure, volume, and moles of a gas.
The four properties of gases (pressure P, volume V.
temperature T, and moles of gas n) are related by a single
expression known as the ideal gas law:
HH
PV = nRT
The variable R is known as the universal gas constant and
has the value R=0.0821 L atm/(mole K). The units of
R dictate the units for all other quantities, so when using this
value of R, use units of atmospheres for pressure, liters for
volume, and kelvins for temperature.
Type here to search
O
▼
Y
Part A
MasteringChemistry: Chapter X
Submit
Part B
Om
Vballoon= Value
μA S →
Pearson MyLab and Masterin X
A balloon is floating around outside your window. The temperature outside is 13 °C, and the air pressure is 0.800 atm. Your neighbor, who
released the balloon, tells you that he filled it with 4.50 moles of gas. What is the volume of gas inside this balloon?
Express your answer to three significant figures and include the appropriate units.
▸ View Available Hint(s)
O
HÅ
Units
MasteringChemistry
?
?
A
A 10.0 L gas cylinder is filled with 6.80 moles of gas. The tank is stored at 11 "C. What is the pressure in the tank?
Express your answer to three significant figures and include the appropriate units.
▸ View Available Hint(s)
x | +
G
Activate Windows
Go to
89°F Partly sunny
< 1 of 7
Review | Constants | Periodic Table
0
9
2:33 PM
7/21/2022
X
***
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

