Learning Goal: To understand the Equipartition Theorem and its implications for the mechanical motion of small objects. In statistical mechanics, thermal energy is the random motion of the microscopic world. The average kinetic or potential energy of each degree of freedom of the microscopic world therefore depends on the temperature. If heat is added, molecules increase their translational and rotational speeds, and the atoms constituting the molecules vibrate with larger amplitude about their equilibrium positions. It is a fact of nature that the energy of each degree of freedom is determined solely by the temperature. The Equipartition Theorem states this quantitatively: The average energy associated with each degree of freedom in a system at absolute temperature T is (1/2)kBT, where kB = 1.38 x 10-23 J/K is Boltzmann's constant. A "degree of freedom" corresponds to any dynamical variable that appears quadratically in the energy. For instance, (1/2)mv₂² is the kinetic energy of a gas particle of mass m with velocity component v₂ along the x axis. The Equipartition Theorem follows from the fundamental postulate of statistical mechanics--that every energetically accessible quantum state of a system has equal probability of being populated, which in turn leads to the Boltzmann distribution for a system in thermal equilibrium. From the standpoint of an introductory physics course, equipartition is best regarded as a principle that is justified by observation. In this problem we first investigate the particle model of an ideal gas. An ideal gas has no interactions among its particles, and so its internal energy is entirely "random" kinetic energy. If we consider the gas as a system, its internal energy is analogous to the energy stored in a spring. If one end of the gas container is fitted with a sliding piston, the pressure of the gas on the piston can do useful work. In fact, the empirically discovered ideal gas law, pV = NkBT, enables us to calculate this pressure. This rule of nature is remarkable in that the value of the mass does not affect the energy (or the pressure) of the gas particles' motion, only the temperature. It provides strong evidence for the validity of the Equipartition Theorem as applied to a particle gas: m(v²) = m(v²) = m(v²) = kBT, where the angle brackets represent the average value of that gas particles if the gas is at an absolute temperature T? Express your answer in terms of T, kB, m, and other given quantities. ► View Available Hint(s) Vz,rms=√√(v²) = Submit ✓ Part B Correct Previous Answers ✓ Now consider the same system: a monatomic gas of particles of mass m, except in three dimensions. Find Urms, the rms speed if the gas is at an absolute temperature T. Express your answer in terms of T, kg, m, and other given quantities. ▸ View Available Hint(s) Urms = √√√(²) = kBT m ✓ Correct ▼ Part C Submit Previous Answers 3kBT m ✓ What is the rms speed vo of molecules in air at 0°C? Air is composed mostly of N₂ molecules, so you may assume that it has molecules of average mass 28.0 x 1.661 x 10-27 kg = 4.65 x 10-26 kg. Express your answer in meters per second, to the nearest integer.
Learning Goal: To understand the Equipartition Theorem and its implications for the mechanical motion of small objects. In statistical mechanics, thermal energy is the random motion of the microscopic world. The average kinetic or potential energy of each degree of freedom of the microscopic world therefore depends on the temperature. If heat is added, molecules increase their translational and rotational speeds, and the atoms constituting the molecules vibrate with larger amplitude about their equilibrium positions. It is a fact of nature that the energy of each degree of freedom is determined solely by the temperature. The Equipartition Theorem states this quantitatively: The average energy associated with each degree of freedom in a system at absolute temperature T is (1/2)kBT, where kB = 1.38 x 10-23 J/K is Boltzmann's constant. A "degree of freedom" corresponds to any dynamical variable that appears quadratically in the energy. For instance, (1/2)mv₂² is the kinetic energy of a gas particle of mass m with velocity component v₂ along the x axis. The Equipartition Theorem follows from the fundamental postulate of statistical mechanics--that every energetically accessible quantum state of a system has equal probability of being populated, which in turn leads to the Boltzmann distribution for a system in thermal equilibrium. From the standpoint of an introductory physics course, equipartition is best regarded as a principle that is justified by observation. In this problem we first investigate the particle model of an ideal gas. An ideal gas has no interactions among its particles, and so its internal energy is entirely "random" kinetic energy. If we consider the gas as a system, its internal energy is analogous to the energy stored in a spring. If one end of the gas container is fitted with a sliding piston, the pressure of the gas on the piston can do useful work. In fact, the empirically discovered ideal gas law, pV = NkBT, enables us to calculate this pressure. This rule of nature is remarkable in that the value of the mass does not affect the energy (or the pressure) of the gas particles' motion, only the temperature. It provides strong evidence for the validity of the Equipartition Theorem as applied to a particle gas: m(v²) = m(v²) = m(v²) = kBT, where the angle brackets represent the average value of that gas particles if the gas is at an absolute temperature T? Express your answer in terms of T, kB, m, and other given quantities. ► View Available Hint(s) Vz,rms=√√(v²) = Submit ✓ Part B Correct Previous Answers ✓ Now consider the same system: a monatomic gas of particles of mass m, except in three dimensions. Find Urms, the rms speed if the gas is at an absolute temperature T. Express your answer in terms of T, kg, m, and other given quantities. ▸ View Available Hint(s) Urms = √√√(²) = kBT m ✓ Correct ▼ Part C Submit Previous Answers 3kBT m ✓ What is the rms speed vo of molecules in air at 0°C? Air is composed mostly of N₂ molecules, so you may assume that it has molecules of average mass 28.0 x 1.661 x 10-27 kg = 4.65 x 10-26 kg. Express your answer in meters per second, to the nearest integer.
Related questions
Question
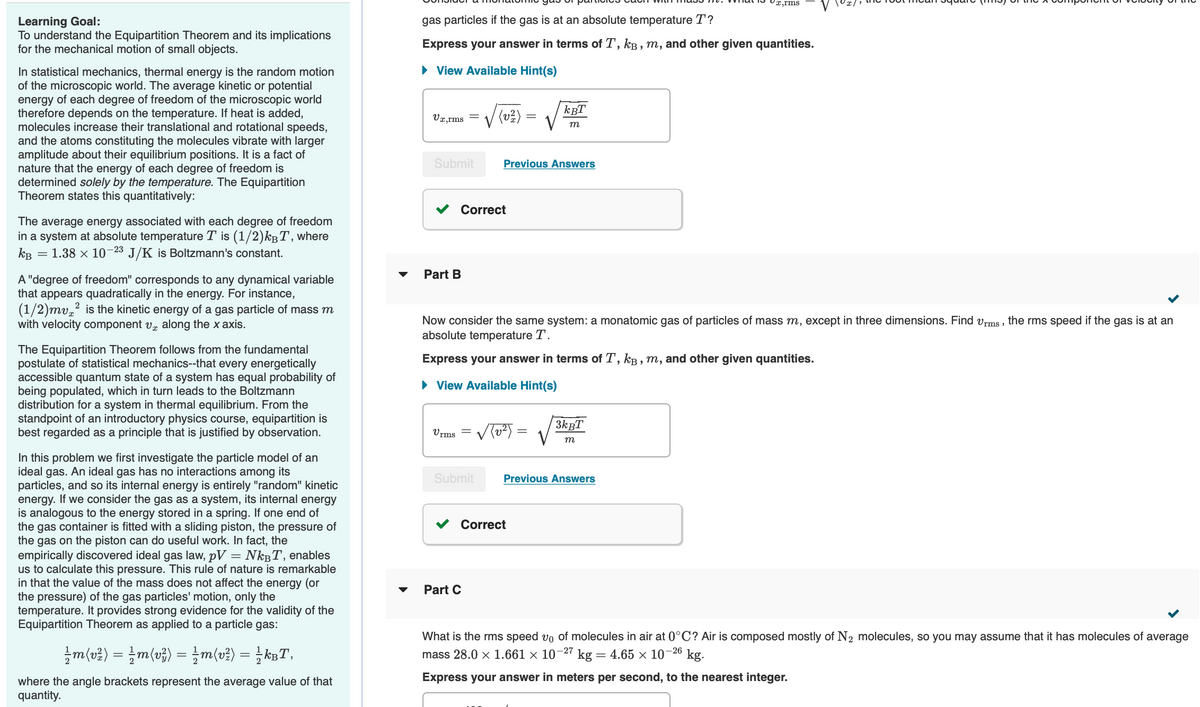
Transcribed Image Text:Learning Goal:
To understand the Equipartition Theorem and its implications
for the mechanical motion of small objects.
In statistical mechanics, thermal energy is the random motion
of the microscopic world. The average kinetic or potential
energy of each degree of freedom of the microscopic world
therefore depends on the temperature. If heat is added,
molecules increase their translational and rotational speeds,
and the atoms constituting the molecules vibrate with larger
amplitude about their equilibrium positions. It is a fact of
nature that the energy of each degree of freedom is
determined solely by the temperature. The Equipartition
Theorem states this quantitatively:
The average energy associated with each degree of freedom
in a system at absolute temperature T is (1/2)k³T, where
KB
=
: 1.38 × 10-2³ J/K is Boltzmann's constant.
A "degree of freedom" corresponds to any dynamical variable
that appears quadratically in the energy. For instance,
(1/2)mv² is the kinetic energy of a gas particle of mass m
with velocity component v along the x axis.
The Equipartition Theorem follows from the fundamental
postulate of statistical mechanics--that every energetically
accessible quantum state of a system has equal probability of
being populated, which in turn leads to the Boltzmann
distribution for a system in thermal equilibrium. From the
standpoint of an introductory physics course, equipartition is
best regarded as a principle that is justified by observation.
In this problem we first investigate the particle model of an
ideal gas. An ideal gas has no interactions among its
particles, and so its internal energy is entirely "random" kinetic
energy. If we consider the gas as a system, its internal energy
is analogous to the energy stored in a spring. If one end of
the gas container is fitted with a sliding piston, the pressure of
the gas on the piston can do useful work. In fact, the
empirically discovered ideal gas law, pV = Nk³T, enables
us to calculate this pressure. This rule of nature is remarkable
in that the value of the mass does not affect the energy (or
the pressure) of the gas particles' motion, only the
temperature. It provides strong evidence for the validity of the
Equipartition Theorem as applied to a particle gas:
½ m (v²) = ½ m(v²) =
=
m(v²) = ½ kBT,
where the angle brackets represent the average value of that
quantity.
gas particles if the gas is at an absolute temperature T?
Express your answer in terms of T, kB, m, and other given quantities.
► View Available Hint(s)
Vx,rms =
Submit
Part B
Correct
(v²/12)
Submit
Vrms √(√²) =
Part C
Previous Answers
=
Now consider the same system: a monatomic gas of particles of mass m, except in three dimensions. Find Urms, the rms speed if the gas is at an
absolute temperature T.
Express your answer in terms of T, kB, m, and other given quantities.
► View Available Hint(s)
КВТ
m
Correct
x,rms
3kBT
m
Previous Answers
What is the rms speed v₁ of molecules in air at 0°C? Air is composed mostly of N₂ molecules, so you may assume that it has molecules of average
mass 28.0 × 1.661 × 10-27 kg = 4.65 × 10-26 kg.
Express your answer in meters per second, to the nearest integer.
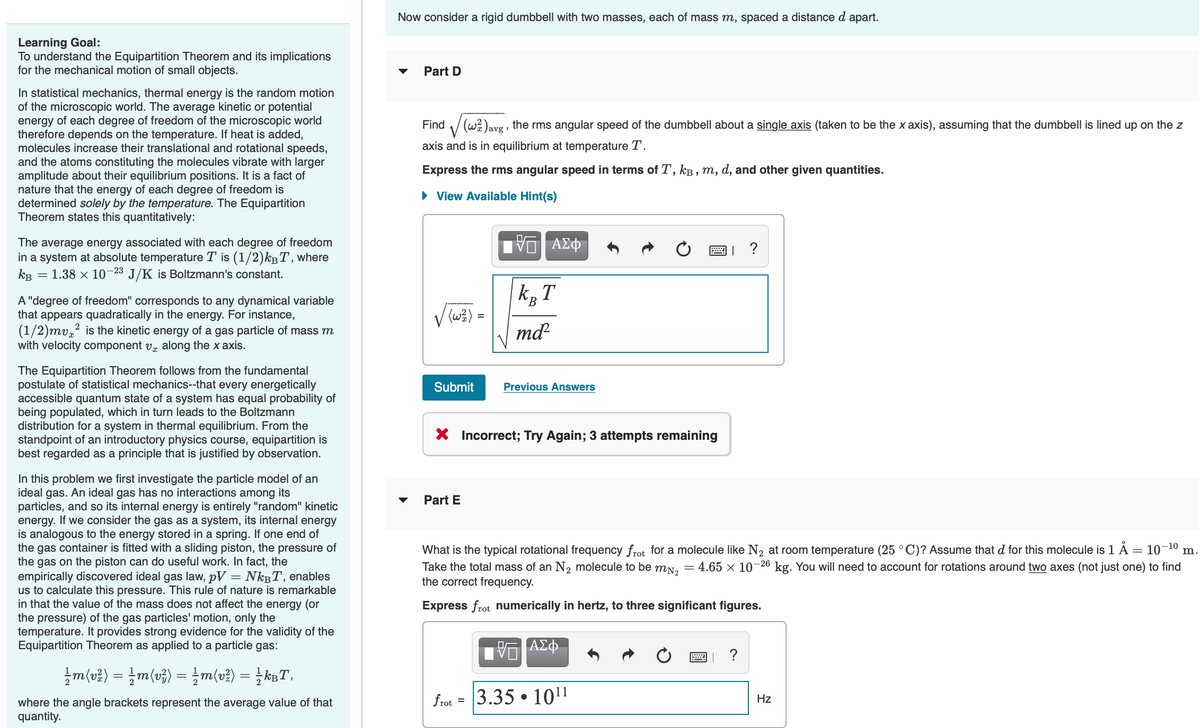
Transcribed Image Text:Learning Goal:
To understand the Equipartition Theorem and its implications
for the mechanical motion of small objects.
In statistical mechanics, thermal energy is the random motion
of the microscopic world. The average kinetic or potential
energy of each degree of freedom of the microscopic world
therefore depends on the temperature. If heat is added,
molecules increase their translational and rotational speeds,
and the atoms constituting the molecules vibrate with larger
amplitude about their equilibrium positions. It is a fact of
nature that the energy of each degree of freedom is
determined solely by the temperature. The Equipartition
Theorem states this quantitatively:
The average energy associated with each degree of freedom
in a system at absolute temperature T is (1/2)k³T, where
kB = 1.38 × 10-23 J/K is Boltzmann's constant.
A "degree of freedom" corresponds to any dynamical variable
that appears quadratically in the energy. For instance,
(1/2)mv² is the kinetic energy of a gas particle of mass m
with velocity component v along the x axis.
The Equipartition Theorem follows from the fundamental
postulate of statistical mechanics--that every energetically
accessible quantum state of a system has equal probability of
being populated, which in turn leads to the Boltzmann
distribution for a system in thermal equilibrium. From the
standpoint of an introductory physics course, equipartition is
best regarded as a principle that is justified by observation.
In this problem we first investigate the particle model of an
ideal gas. An ideal gas has no interactions among its
particles, and so its internal energy is entirely "random" kinetic
energy. If we consider the gas as a system, its internal energy
is analogous to the energy stored in a spring. If one end of
the gas container is fitted with a sliding piston, the pressure of
the gas on the piston can do useful work. In fact, the
empirically discovered ideal gas law, pV NKBT, enables
us to calculate this pressure. This rule of nature is remarkable
in that the value of the mass does not affect the energy (or
the pressure) of the gas particles' motion, only the
temperature. It provides strong evidence for the validity of the
Equipartition Theorem as applied to a particle gas:
=
{m(v²) = {m(v²) = ½m(v²) = ½ kbT,
where the angle brackets represent the average value of that
quantity.
Now consider a rigid dumbbell with two masses, each of mass m, spaced a distance d apart.
Part D
Find (w) avg, the rms angular speed of the dumbbell about a single axis (taken to be the x axis), assuming that the dumbbell is lined up on the z
axis and is in equilibrium at temperature T.
Express the rms angular speed in terms of T, kB, m, d, and other given quantities.
► View Available Hint(s)
√√(w ² ) =
Submit Previous Answers
—| ΑΣΦΑ
k₂ T
B
md²2
X Incorrect; Try Again; 3 attempts remaining
Part E
frot =
What is the typical rotational frequency frot for a molecule like N₂ at room temperature (25 °C)? Assume that d for this molecule is 1 Å = 10–¹⁰ m.
Take the total mass of an N₂ molecule to be m№₂ = 4.65 × 10-26 kg. You will need to account for rotations around two axes (not just one) to find
the correct frequency.
Express frot numerically in hertz, to three significant figures.
ΨΕ ΑΣΦ
3.35 101¹1
●
?
?
Hz
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
