Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter6: Electronic Structure And Periodic Properties Of Elements
Section: Chapter Questions
Problem 82E: Of the five elements Al, Cl, I, Na, Rb, which has the most exothermic reaction? (E represents an...
Related questions
Question
Transcribed Image Text:Course Home
(Chapter 6
Problem 6.148 - Enhanced - with Feedback
You may want to reference
(Pages 170 - 173) Section 6.1 while completing this
problem.
P.
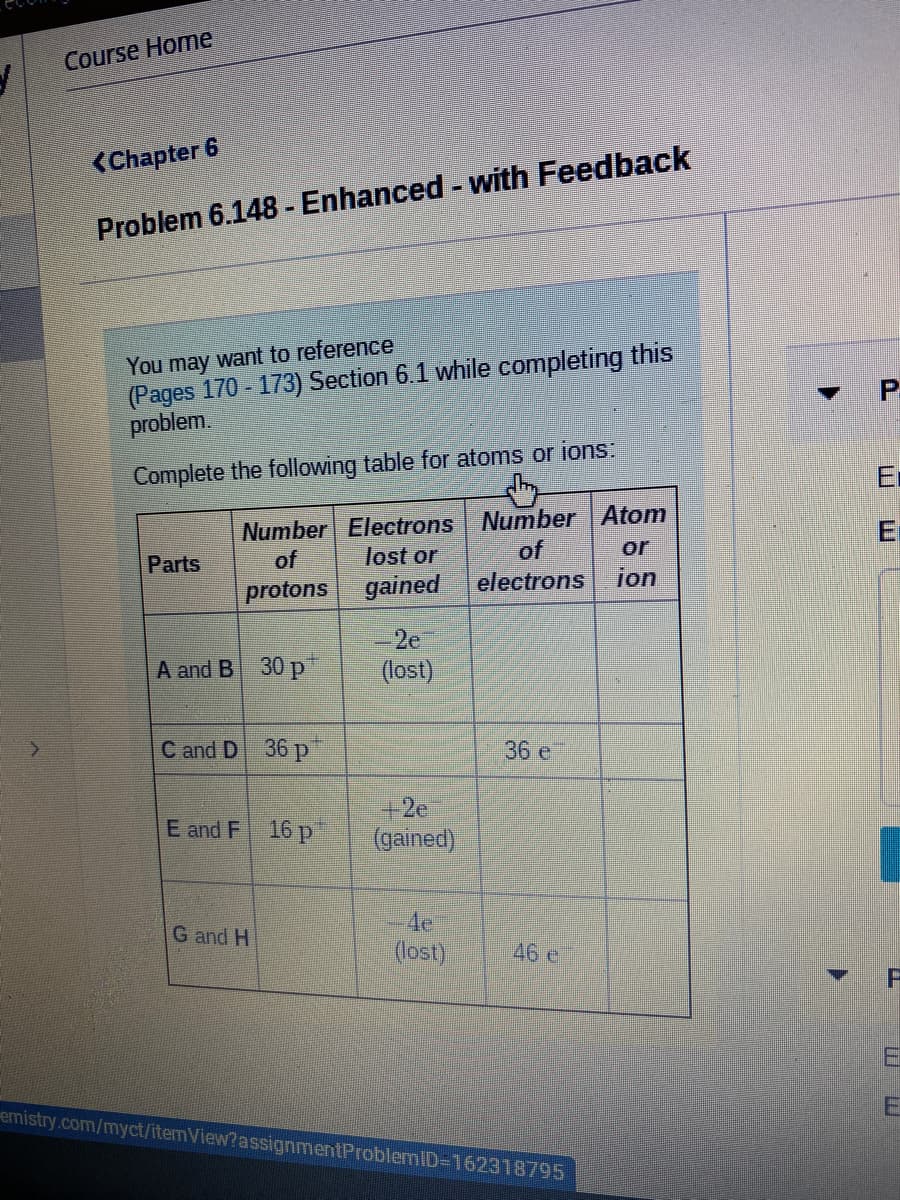
Complete the following table for atoms or ions:
Number Electrons Number Atom
lost or
of
electrons
Parts
of
or
protons gained
ion
-2e
A and B 30 р
(lost)
C and D 36 p
36 e
+2e
(gained)
E and F 16 p
G and H
4e.
(lost)
46 е
emistry.com/myct/itemView?assignmentProblemID3D162318795
山
E.
Transcribed Image Text:Review Constants |
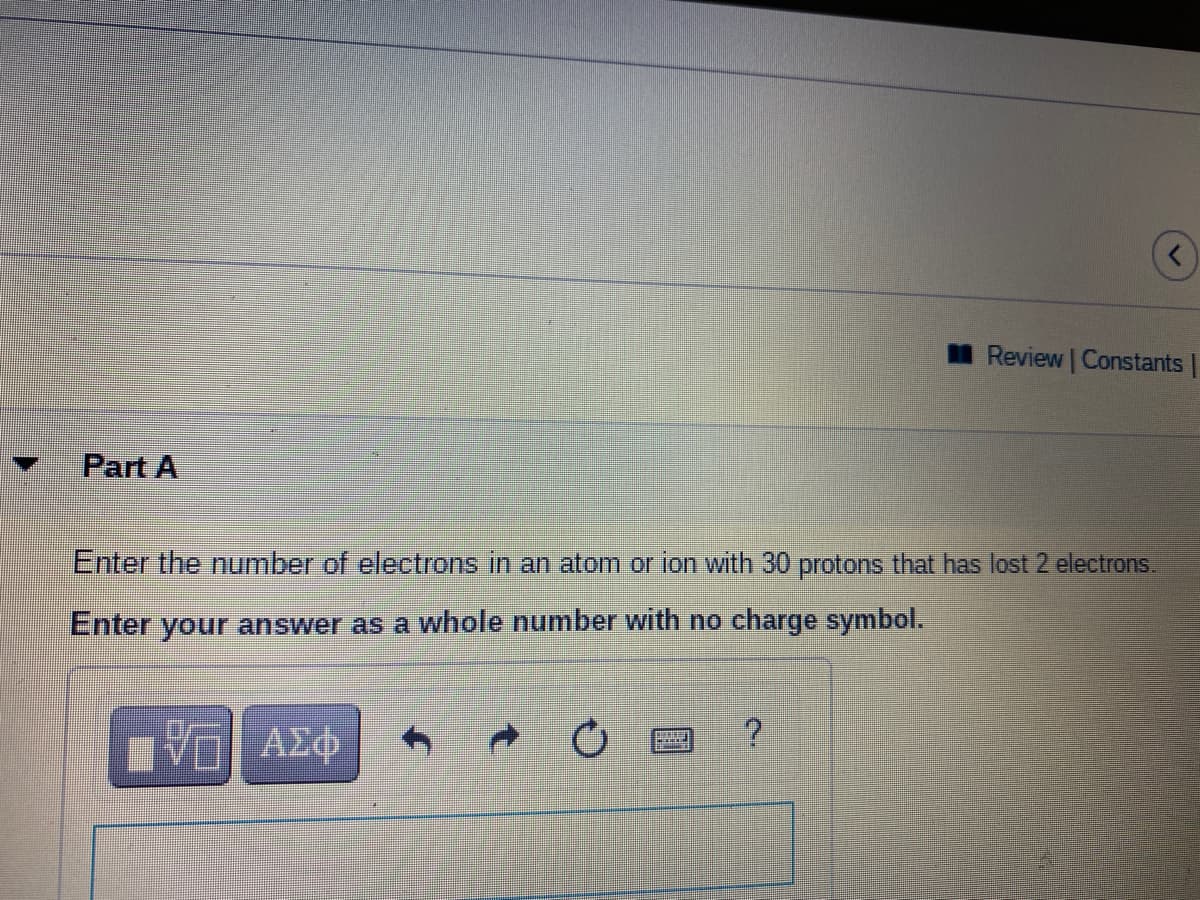
Part A
Enter the number of electrons in an atom or ion with 30 protons that has lost 2 electrons.
Enter your answer as a whole number with no charge symbol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning