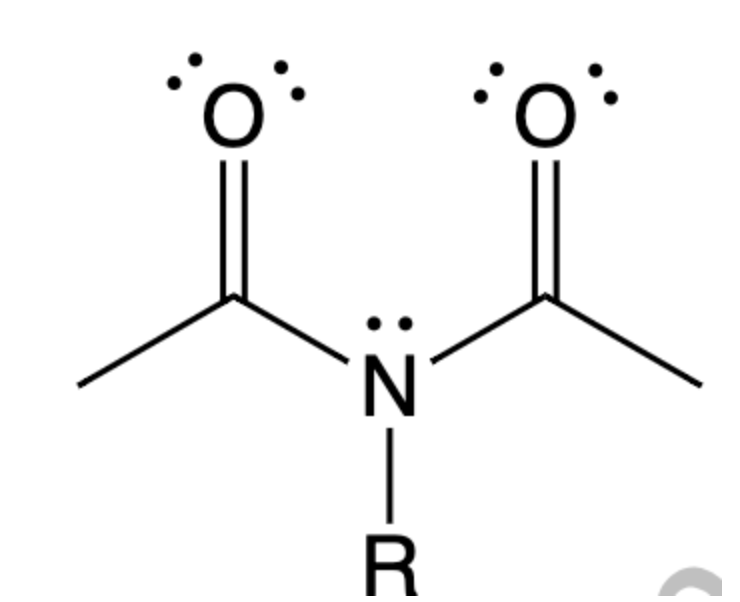
Look at the imide, the nitrogen analog of an anhydride below: a) For a neutral imide, will the carbonyl carbon be more (easier to attack) or less (harder to attack) electrophilic than the C of a neutral anhydride? Talk about electronegativties of Nitrogen (imide) vs Oxygen (anyhydride) b) If the imide is hydrolyzed under acidic conditions, which atom, one of the oxygens or the nitrogen, will be protonated? Explain your answer. Talk about pka and keq.
Look at the imide, the nitrogen analog of an anhydride below: a) For a neutral imide, will the carbonyl carbon be more (easier to attack) or less (harder to attack) electrophilic than the C of a neutral anhydride? Talk about electronegativties of Nitrogen (imide) vs Oxygen (anyhydride) b) If the imide is hydrolyzed under acidic conditions, which atom, one of the oxygens or the nitrogen, will be protonated? Explain your answer. Talk about pka and keq.
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Look at the imide, the nitrogen analog of an anhydride below:
a) For a neutral imide, will the carbonyl carbon be more (easier to attack) or
less (harder to attack) electrophilic than the C of a neutral anhydride? Talk about electronegativties of Nitrogen (imide) vs Oxygen (anyhydride)
b) If the imide is hydrolyzed under acidic conditions, which atom, one of the
oxygens or the nitrogen, will be protonated? Explain your answer. Talk about pka and keq.
Transcribed Image Text:PIN:
-
.O.
O.:
=0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
shouldn't part b be oxygen due to resonance and stability?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

