m2, T, T2 m. T; =f m2 m3
International Edition---engineering Mechanics: Statics, 4th Edition
4th Edition
ISBN:9781305501607
Author:Andrew Pytel And Jaan Kiusalaas
Publisher:Andrew Pytel And Jaan Kiusalaas
Chapter1: Introduction To Statics
Section: Chapter Questions
Problem 1.31P: Resolve the 360-lb force into components along the cables AB and AC. Use =60 and =40.
Related questions
Question
The wall separating the two compartments is removed and the two gases are allowed to mix. Assuming constant specific heats, find the simplest expression for the mixture temperature written in the form where m3 and T3 are the mass and temperature of the final mixture, respectively
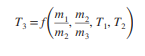
Transcribed Image Text:m2, T, T2
m.
T; =f
m2 m3
Expert Solution
Step 1
Given:
- The mass of the first gas is m1.
- The mass of the second gas is m2.
- The temperature of both the gases is T1 and T2.
- The mass and temperature of the mixture of the gases are m3 and T3.
The relation between the mass of mixture and gases is,
The expression for the energy equation is,
Here Ein and Eout are the energies inside and outside the system and ∆Esystem is the change in energy of the system.
Since it’s in closed system energy transferred outside the system is zero and then change in internal energy will be zero.
Here ∆U is the change in internal energy.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

International Edition---engineering Mechanics: St…
Mechanical Engineering
ISBN:
9781305501607
Author:
Andrew Pytel And Jaan Kiusalaas
Publisher:
CENGAGE L

International Edition---engineering Mechanics: St…
Mechanical Engineering
ISBN:
9781305501607
Author:
Andrew Pytel And Jaan Kiusalaas
Publisher:
CENGAGE L