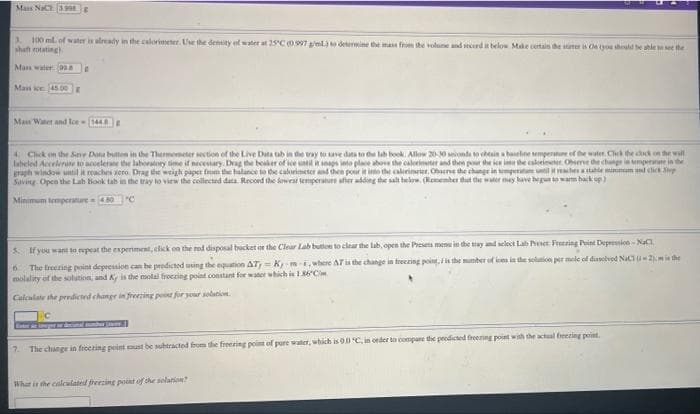
Mas NaC 39 3. 100 ml. of water is already in the calorimeter. Use the denity of water at 25'C0.997 ml determine the mas fm the volune and mrd it belo Make certain the ner is Ce tyou should be ahle t see the shaft rotating. Mas waler asa Mass oe 45.00E Man Watet and loe (144 4 Chck on the Sne Data buten in the Thermometer section of the Live Data tab in dhe vay to ave data to de lab book. Allow 2010 ionda tein a haelne empenatue of the water. Click the cck on the wall labeled Accelerate to acoelerne the laboratory time if necestary. Dvag the beaher of lee unl i seags into place above the calorinter and then pour the ice ima the caleieeer. Oierve the change in empere in the graph window until it reaches sero. Drag the weigh paper from the halance to de calorimeter and then pour it io the calerimrter, Obene the change in temperatune uilirechetale minim nd elick p Saving Open the Lab Book tab in the tray to view the collected data. Record the fowrat temperature afher adding the salt below. (Remenber dat e water may have hegun to wam hak up) Minimum temperature 0 "C 5. If you want to repeat the esperiment, click on the red disposal bucket or the Clear Lab buton to clear the lab, open the Presets menu in the tay and select Lab eer Freering Point Depression-NaC The freering point depression can be prodicted using the equation AT, = Kmi, where AT is the change in freezing point, i the munter of is in de solution per mole of diaslved NaCu2).mie the molality of the solution, and A is the motal froczing point constant for water which is I86C Calculate the prediced changr in frezing poist for your solation. 7. The change in freeting point aust be subtracted fom the freeting point of pure water, which is O0 C, in order to compane the prodicted freening point with the actual freezing point. Whar is the calculated freezing point of she solation?
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Chemistry
Step by step
Solved in 3 steps with 3 images









