Mass number 238 236 234 232 230 228 226 224 222 220 218 216 214 212 210 208 206 204 Pb-Bi Po Po Pb Bi Po Pb Part A Each blue arrow represents decay by alpha emission. 81 82 83 84 85 Tl Pb Bi Po At Rn Ra Each red arrow represents decay by beta emission Th Pa U E U Th 87 88 89 90 86 Rn Fr Ra Ac Th Atomic number Nuclear decay chain for uranium-238. The decay continues until the stable nucleus 206 Pb is formed. 91 92 93 Pa U Np Write the nuclear equation for the step shown for the first decay of U. Express your answer as a nuclear equation including mass numbers and atomic numbers.
Mass number 238 236 234 232 230 228 226 224 222 220 218 216 214 212 210 208 206 204 Pb-Bi Po Po Pb Bi Po Pb Part A Each blue arrow represents decay by alpha emission. 81 82 83 84 85 Tl Pb Bi Po At Rn Ra Each red arrow represents decay by beta emission Th Pa U E U Th 87 88 89 90 86 Rn Fr Ra Ac Th Atomic number Nuclear decay chain for uranium-238. The decay continues until the stable nucleus 206 Pb is formed. 91 92 93 Pa U Np Write the nuclear equation for the step shown for the first decay of U. Express your answer as a nuclear equation including mass numbers and atomic numbers.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section: Chapter Questions
Problem 62GQ
Related questions
Question
100%
15) please see picture
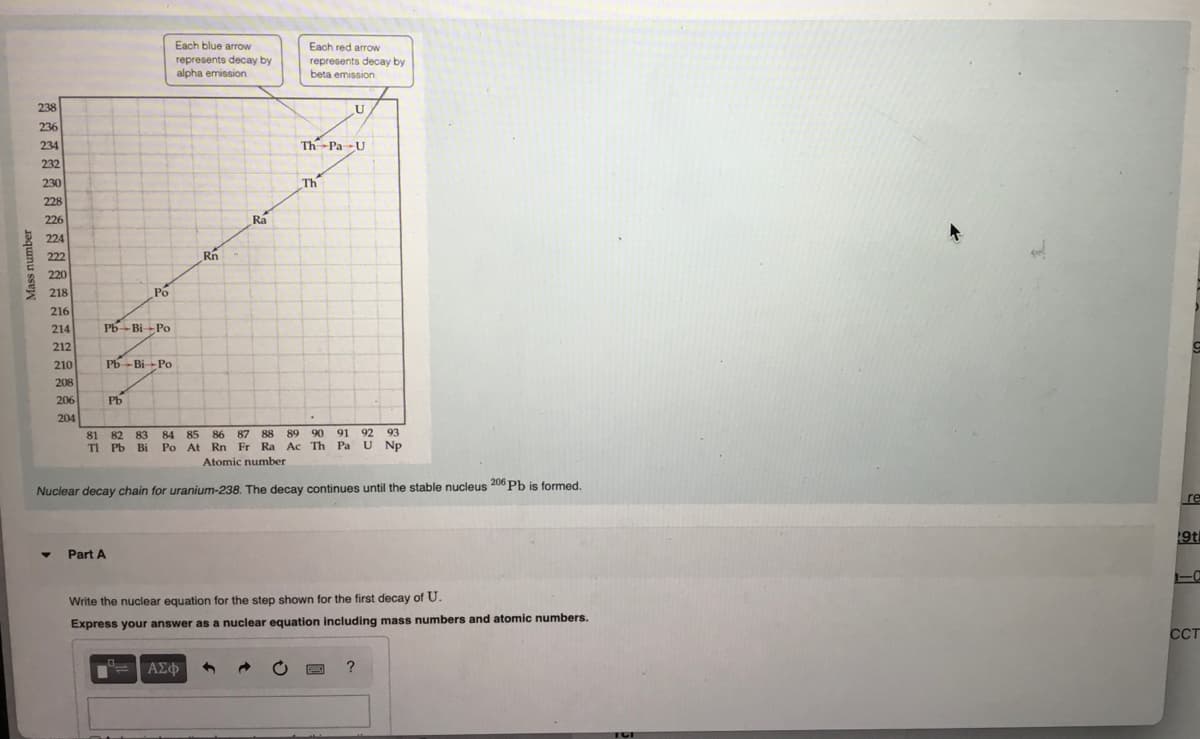
Transcribed Image Text:Mass number
238
236
234
232
230
228
226
224
222
220
218
216
214
212
210
208
206
204
Pb Bi Po
Po
Pb Bi Po
Pb
Part A
Each blue arrow
represents decay by
alpha emission.
Rn
Ra
ΑΣΦ
Each red arrow
represents decay by
beta emission.
U
Th Pa U
Th
81
82 83 84 85 86 87 88 89 90 91 92 93
T1 Pb Bi Po At Rn Fr Ra Ac Th Pa U Np
Atomic number
Nuclear decay chain for uranium-238. The decay continues until the stable nucleus 206 Pb is formed.
Write the nuclear equation for the step shown for the first decay of U.
Express your answer as a nuclear equation including mass numbers and atomic numbers.
?
TOI
re
9tl
CCT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning