Match the following aqueous solutions with the appropriate letter from the column on the right. 1.0.12 m Mg(CH3COO); A. Lowest freezing point 2. 0.16 m FeSO4 B. Second lowest freezing point |3. 8.0x102 m Al2(So,)3 C. Third lowest freezing point 4.0.34 m Urea(nonelectrolyte) D. Highest freezing point
Match the following aqueous solutions with the appropriate letter from the column on the right. 1.0.12 m Mg(CH3COO); A. Lowest freezing point 2. 0.16 m FeSO4 B. Second lowest freezing point |3. 8.0x102 m Al2(So,)3 C. Third lowest freezing point 4.0.34 m Urea(nonelectrolyte) D. Highest freezing point
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.139QP
Related questions
Question
I don’t know how to do this question
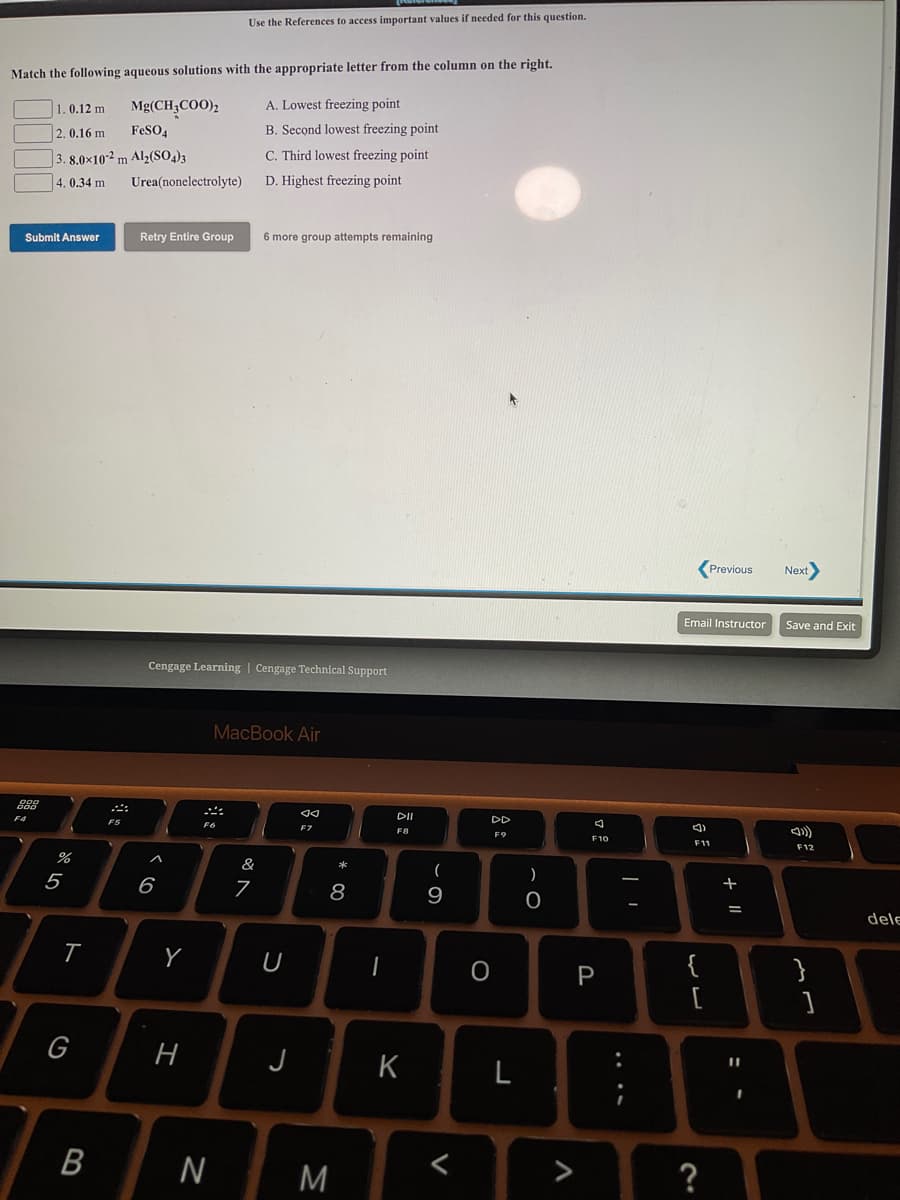
Transcribed Image Text:Use the References to access important values if needed for this question.
Match the following aqueous solutions with the appropriate letter from the column on the right.
1.0.12 m
Mg(CH;COO)2
A. Lowest freezing point
2. 0.16 m
FeSO,
B. Second lowest freezing point
|3. 8.0×10-² m Al2(SO,)3
C. Third lowest freezing point
4. 0.34 m
Urea(nonelectrolyte)
D. Highest freezing point
Submit Answer
Retry Entire Group
6 more group attempts remaining
Previous
Next>
Email Instructor
Save and Exit
Cengage Learning | Cengage Technical Support
MacBook Air
DI
F5
F8
F10
F11
F12
&
*
5
6
7
8
9
dele
U
{
[
P
}
G
J
K
L
?
....
V
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
