Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed to the process contains 32.0 mole% CO, 64.0% H2, and 4.0% N2. This stream is mixed with a recycle stream in a ratio 5 mol recycle/1 mol fresh feed to produce the feed to the reactor, which contains 13.0 mole % N2. A low single-pass conversion is attained in the reactor. The reactor effluent goes to a condenser from which two streams emerge: a liquid product stream containing essentially all the methanol formed in the reactor, and a gas stream containing all the CO, H2, and N2 leaving the reactor. The gas stream is split into two fractions: one is removed from the process as a purge stream, and the other is the recycle stream that combines with the fresh feed to the reactor. (a) Write the basis of calculation. (b) Draw and label a flowchart. (c) Write the chemical reaction equation. (d) Carry out a degree of freedom analysis based on molecular species balances, atomic species balances and extent of reaction. (e) Setup and solve the mass balance equations for determining the production rate of methanol, the molar flow rate and composition of the purge gas, and the overall and single-pass conversion. b7 1o0 kmol/h FF Cнон: Mz Kmoyh Reactor condenser N= 43 KmgL' Coi 32 kmoyh Н, 64 Кeyh Nz=4 Kmol/h R =500 kmouh Purge Co IHz
Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed to the process contains 32.0 mole% CO, 64.0% H2, and 4.0% N2. This stream is mixed with a recycle stream in a ratio 5 mol recycle/1 mol fresh feed to produce the feed to the reactor, which contains 13.0 mole % N2. A low single-pass conversion is attained in the reactor. The reactor effluent goes to a condenser from which two streams emerge: a liquid product stream containing essentially all the methanol formed in the reactor, and a gas stream containing all the CO, H2, and N2 leaving the reactor. The gas stream is split into two fractions: one is removed from the process as a purge stream, and the other is the recycle stream that combines with the fresh feed to the reactor. (a) Write the basis of calculation. (b) Draw and label a flowchart. (c) Write the chemical reaction equation. (d) Carry out a degree of freedom analysis based on molecular species balances, atomic species balances and extent of reaction. (e) Setup and solve the mass balance equations for determining the production rate of methanol, the molar flow rate and composition of the purge gas, and the overall and single-pass conversion. b7 1o0 kmol/h FF Cнон: Mz Kmoyh Reactor condenser N= 43 KmgL' Coi 32 kmoyh Н, 64 Кeyh Nz=4 Kmol/h R =500 kmouh Purge Co IHz
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
1.Carry out a degree of freedom analysis based on molecular species balances,atomic species balances and extent of reaction.(all of this)
Transcribed Image Text:Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed
to the process contains 32.0 mole% CO, 64.0% H2, and 4.0% N2. This stream is mixed with a recycle
stream in a ratio 5 mol recycle/1 mol fresh feed to produce the feed to the reactor, which contains
13.0 mole % N2. A low single-pass conversion is attained in the reactor. The reactor effluent goes
to a condenser from which two streams emerge: a liquid product stream containing essentially all
the methanol formed in the reactor, and a gas stream containing all the CO, H2, and N2 leaving the
reactor. The gas stream is split into two fractions: one is removed from the process as a purge stream,
and the other is the recycle stream that combines with the fresh feed to the reactor.
(a) Write the basis of calculation.
(b) Draw and label a flowchart.
(c) Write the chemical reaction equation.
(d) Carry out a degree of freedom analysis based on molecular species balances,
atomic species balances and extent of reaction.
(e) Setup and solve the mass balance equations for determining the production rate
of methanol, the molar flow rate and composition of the purge gas, and the overall and
single-pass conversion.
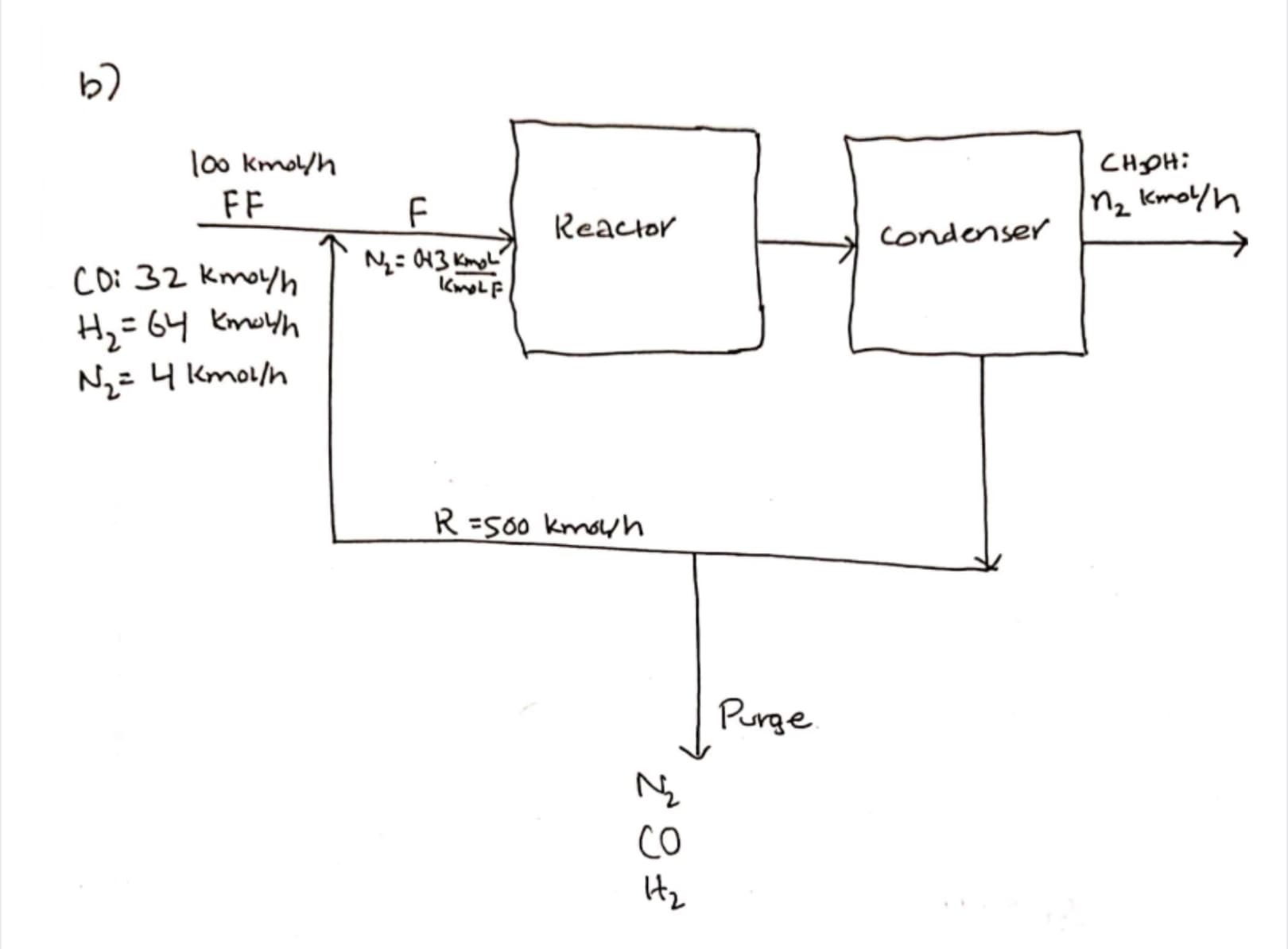
Transcribed Image Text:b7
1o0 kmol/h
FF
Cнон:
Mz Kmoyh
Reactor
condenser
N= 43 KmgL'
Coi 32 kmoyh
Н, 64 Кeyh
Nz=4 Kmol/h
R =500 kmouh
Purge
Co
IHz
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 14 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The