MISSED THIS? Read Sections 4.3 (Pages 145- 149), 5.3 (Pages 173 - 174) ; Watch KCV 4.3, IWE 4.6, IWE 5.4. Part A A 28.4 mL sample of a 1.68M potassium chloride solution is mixed with 14.7 mL of a 0.900 M lead(1I) nitrate solution and this precipitation reaction occurs: Determine the limiting reactant. 2KCI(aq) + Pb(NO3)2(aq) → PbC2 (s) + 2KN03(aq) Express your answer as a chemical formula. The solid PbCl, is collected, dried, and found to have a mass of 2.49 g. Determine the limiting reactant, the theoretical yield, and the percent yield. • View Available Hint(s) ΑΣφ OA chemical reaction does not occur for this question. Submit Part B Complete previous part(s) Part C Complete previous part(s)
MISSED THIS? Read Sections 4.3 (Pages 145- 149), 5.3 (Pages 173 - 174) ; Watch KCV 4.3, IWE 4.6, IWE 5.4. Part A A 28.4 mL sample of a 1.68M potassium chloride solution is mixed with 14.7 mL of a 0.900 M lead(1I) nitrate solution and this precipitation reaction occurs: Determine the limiting reactant. 2KCI(aq) + Pb(NO3)2(aq) → PbC2 (s) + 2KN03(aq) Express your answer as a chemical formula. The solid PbCl, is collected, dried, and found to have a mass of 2.49 g. Determine the limiting reactant, the theoretical yield, and the percent yield. • View Available Hint(s) ΑΣφ OA chemical reaction does not occur for this question. Submit Part B Complete previous part(s) Part C Complete previous part(s)
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.36QAP
Related questions
Question
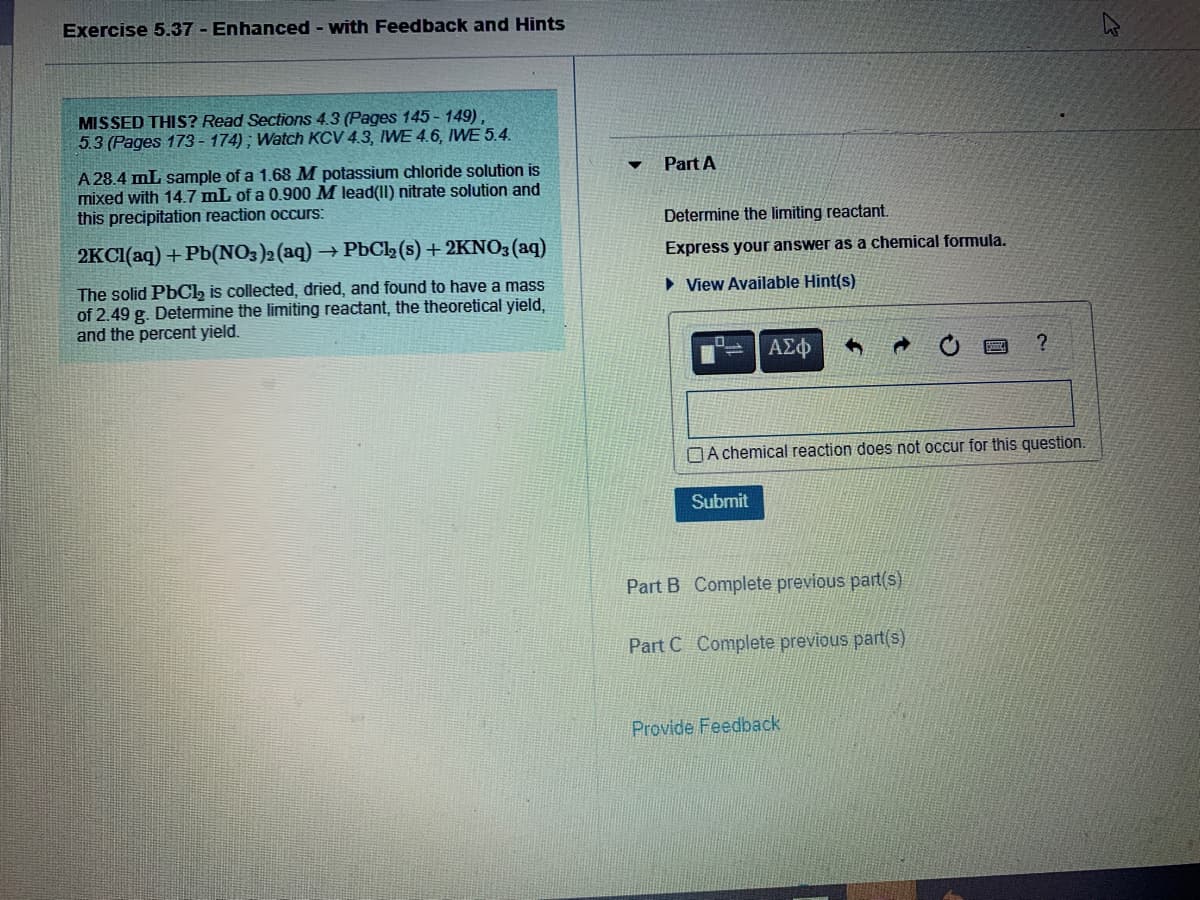
Transcribed Image Text:Exercise 5.37 - Enhanced - with Feedback and Hints
MISSED THIS? Read Sections 4.3 (Pages 145 - 149),
5.3 (Pages 173 - 174) ; Watch KCV 4.3, WE 4.6, IWE 5.4.
Part A
A 28.4 mL sample of a 1.68M potassium chloride solution is
mixed with 14.7 mL of a 0.900 M lead(II) nitrate solution and
this precipitation reaction occurs:
Determine the limiting reactant.
2KCI(aq) + Pb(NO3)2 (aq)
- PbCl2 (s) + 2KNO3 (aq)
Express your answer as a chemical formula.
The solid PbCl, is collected, dried, and found to have a mass
of 2.49 g. Determine the limiting reactant, the theoretical yield,
and the percent yield.
• View Available Hint(s)
OA chemical reaction does not occur for this question.
Submit
Part B Complete previous part(s)
Part C Complete previous part(s)
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

