More strong base is added until the equivalence point is reached. What is the pH of this solution at the equivalence point if the total volume is 36.0 mL ? Express the pH numerically to two decimal places. • View Available Hint(s) ? pH =
More strong base is added until the equivalence point is reached. What is the pH of this solution at the equivalence point if the total volume is 36.0 mL ? Express the pH numerically to two decimal places. • View Available Hint(s) ? pH =
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 8ALQ: You have a solution of the weak acid HA and add some of the salt NaA to it. What are the major...
Related questions
Question
Part A. may help to answer part b

Transcribed Image Text:A certain weak acid, HA, with a K, value of 5.61 × 10-6, is titrated
with NaOH.
Part A
A solution is made by titrating 8.00 mmol (millimoles) of HA and
1.00 mmol of the strong base. What is the resulting pH?
Express the pH numerically to two decimal places.
View Available Hint(s)
pH = 4.40
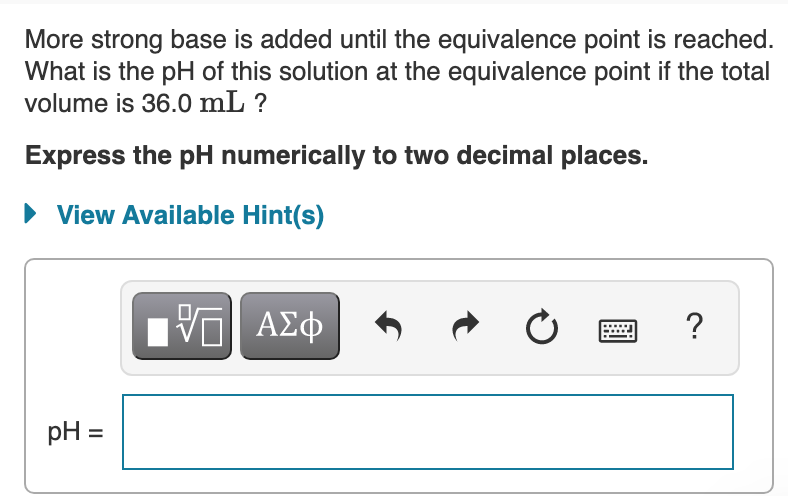
Transcribed Image Text:More strong base is added until the equivalence point is reached.
What is the pH of this solution at the equivalence point if the total
volume is 36.0 mL ?
Express the pH numerically to two decimal places.
• View Available Hint(s)
?
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
