Morpholine is a common additive used for pH adjustment in fossil fuel and nuclear power plant steam systems to provide corrosion protection. How many milliliters of 0.1130 M HBr should be added to 52.20 mL of 0.01340 M morpholine to give a pH of 8.00?
Morpholine is a common additive used for pH adjustment in fossil fuel and nuclear power plant steam systems to provide corrosion protection. How many milliliters of 0.1130 M HBr should be added to 52.20 mL of 0.01340 M morpholine to give a pH of 8.00?
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 13ALQ: Chlorine exists mainly as two isotopes, 37Cl and 33Cl. Which is more abundant? How do you know?
Related questions
Question
Morpholine is a common additive used for pH adjustment in fossil fuel and nuclear power plant steam systems to provide corrosion protection. How many milliliters of 0.1130 M HBr should be added to 52.20 mL of 0.01340 M morpholine to give a pH of 8.00?
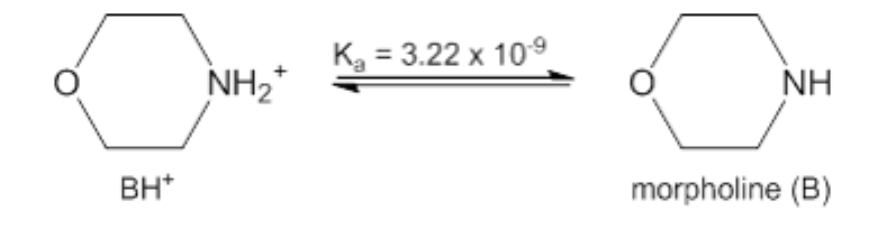
Transcribed Image Text:Kg = 3.22 x 109
NH,*
NH
BH*
morpholine (B)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
