Mr. Romeo has 102 grams of Methane, CH4. How many moles does he have available to burn? Part 2: Write out the balanced formula for the combustion of Methane.
Mr. Romeo has 102 grams of Methane, CH4. How many moles does he have available to burn? Part 2: Write out the balanced formula for the combustion of Methane.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.2: Balancing Chemical Equations
Problem 3.1CYU: (a) Butane gas, C4H10, can burn completely in air [use O2(g) as the other reactant] to give carbon...
Related questions
Question
A BCA table would be nice if you guys could do it, but if you need me to ask a separate question with the balanced formula I will. This is part of a pre test that’s not graded, I would like to know how to solve this.
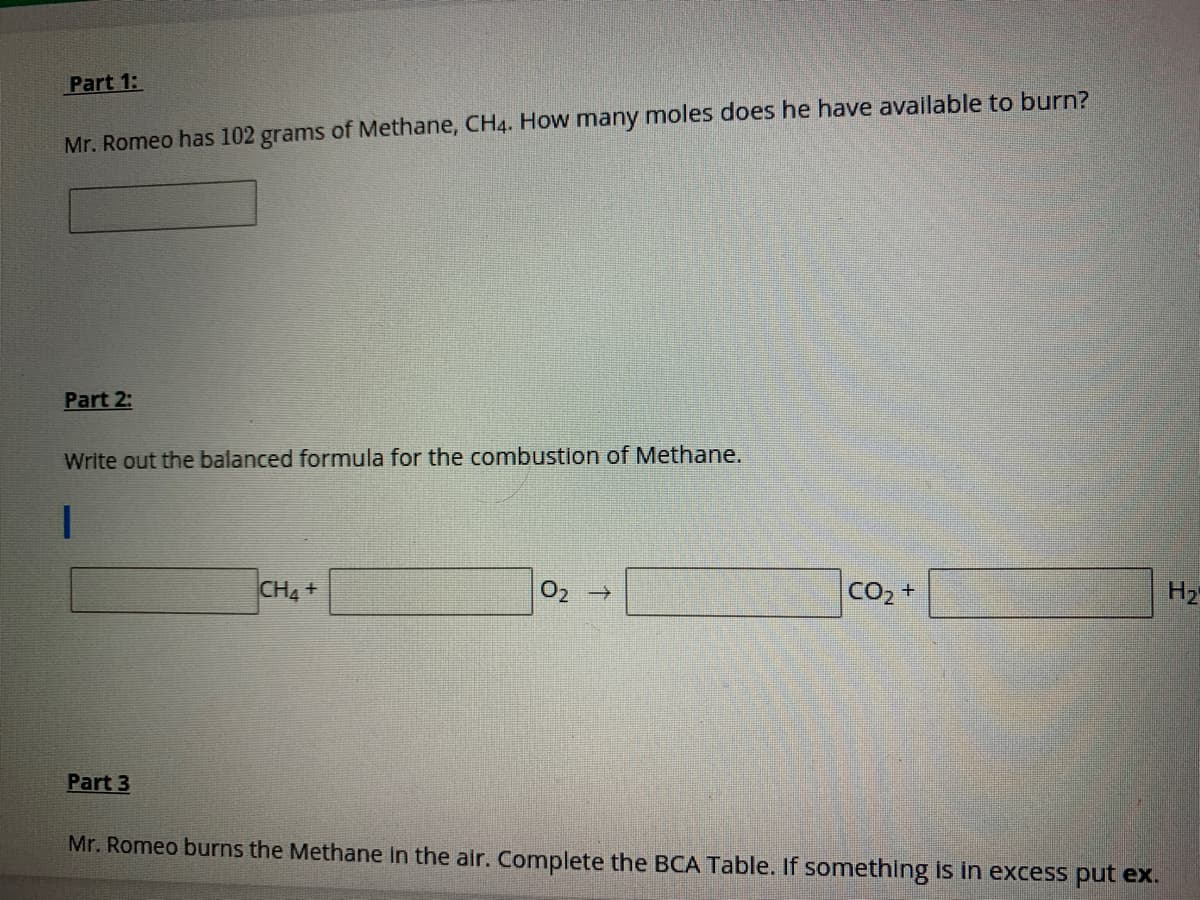
Transcribed Image Text:Part 1:
Mr. Romeo has 102 grams of Methane, CH4. How many moles does he have available to burn?
Part 2:
Write out the balanced formula for the combustion of Methane.
CH4+
CO2 +
H2
Part 3
Mr. Romeo burns the Methane in the air. Complete the BCA Table. If something is in excess put ex.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning