must consist of a single step. By adding a catalyst to the reaction, the reaction rate will increase. 1. True 2. False Raising the temperature will cause the reaction rate to decrease. If the temperature is increased, the activation energy will decrease. The reaction is second- order overall.
must consist of a single step. By adding a catalyst to the reaction, the reaction rate will increase. 1. True 2. False Raising the temperature will cause the reaction rate to decrease. If the temperature is increased, the activation energy will decrease. The reaction is second- order overall.
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 3RQ: One experimental procedure that can be used to determine the rate law of a reaction is the method of...
Related questions
Question
4
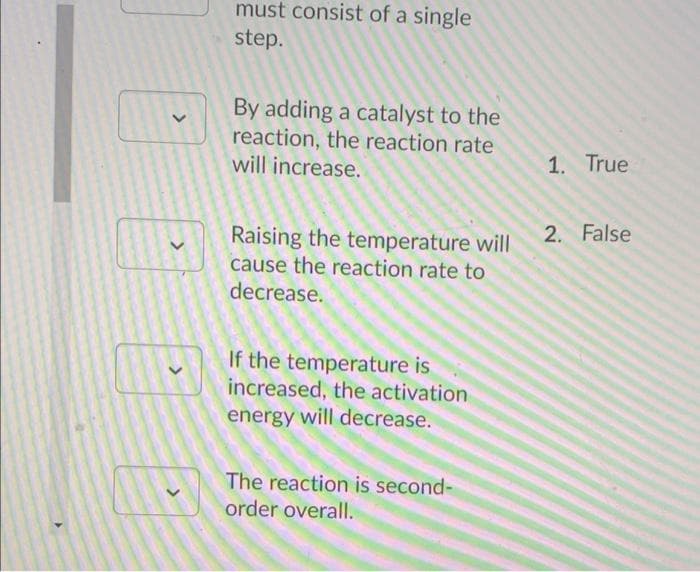
Transcribed Image Text:must consist of a single
step.
By adding a catalyst to the
reaction, the reaction rate
will increase.
1. True
2. False
Raising the temperature will
cause the reaction rate to
decrease.
If the temperature is
increased, the activation
energy will decrease.
The reaction is second-
order overall.
![Consider the gas-phase reaction to form hydrogen iodide, HI. The rate law has been
determined experimentally to be Rate = k [H2] [121.
%3D
H2(g) + I2(g)-2 HI(g)
Identify each statement as being either true or false.
If the concentration of both
reactants is doubled, then
the rate will be doubled.
The reaction mechanism
must consist of a single
step.
By adding a catalyst to the
reaction, the reaction rate
will increase
1. True](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F63197d1f-41f9-4e90-82e9-654b37b65776%2Feb38ac87-aa82-484a-b5e1-f112d19ce795%2F9e5kt7k_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Consider the gas-phase reaction to form hydrogen iodide, HI. The rate law has been
determined experimentally to be Rate = k [H2] [121.
%3D
H2(g) + I2(g)-2 HI(g)
Identify each statement as being either true or false.
If the concentration of both
reactants is doubled, then
the rate will be doubled.
The reaction mechanism
must consist of a single
step.
By adding a catalyst to the
reaction, the reaction rate
will increase
1. True
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax