Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter11: Atomic Mass Spectrometry
Section: Chapter Questions
Problem 11.5QAP
Related questions
Question
I have to solve for the values of A and B in this equation. We were told to solve it as two equations, two unknowns using the densities and viscosities of acetone and water and I'm not sure how to approach this. The density for water was 0.995g/mL and for acetone it was 0.791 g/ml. The viscosity of water was 0.892 mpas and for acetone it was 0.295 mpas. The temperature this occurred at was 24.9 degree Celsius.
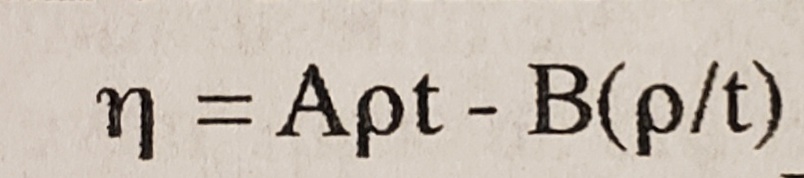
Transcribed Image Text:n=DApt - B(p/t)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
