Name the following compound: Mi₂S Treat Bd. Mi. Wf, Pe, and Im as NONMETALS! Mi is element 147 on the special periodic table. For the toolbar, press ALT+F10(POYO ALT+EN4510 (Mac)
Name the following compound: Mi₂S Treat Bd. Mi. Wf, Pe, and Im as NONMETALS! Mi is element 147 on the special periodic table. For the toolbar, press ALT+F10(POYO ALT+EN4510 (Mac)
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section: Chapter Questions
Problem 28CR
Related questions
Question
Name the compound:
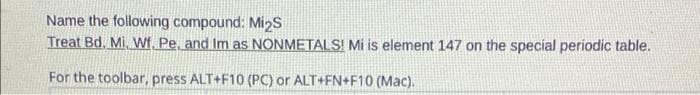
Transcribed Image Text:Name the following compound: Mi₂S
Treat Bd. Mi, Wf, Pe, and Im as NONMETALS! Mi is element 147 on the special periodic table.
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
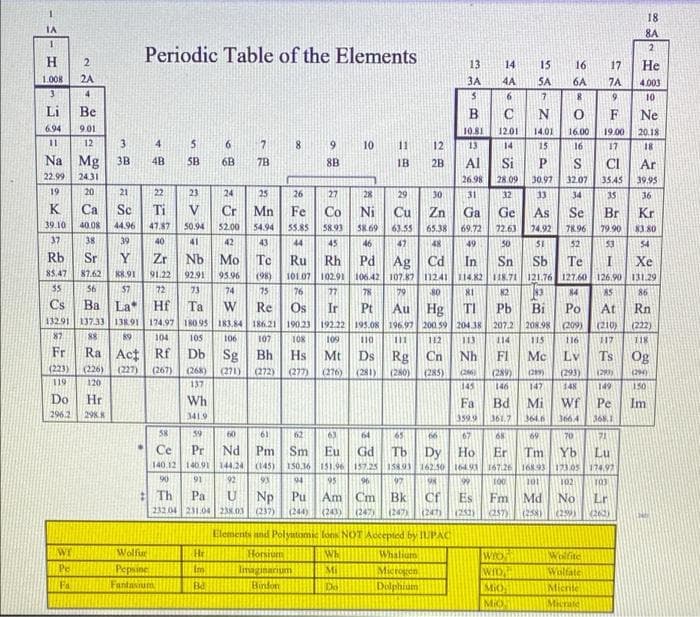
Transcribed Image Text:18
8A
1
2
Periodic Table of the Elements
H
2
13 14
15
16
17
He
1.008 2A
3A
4A 5A
6A
7A
4.003
3
4
5
6
7
8
9
10
Li
Be
B
C N
0
F
Ne
6.94
9.01
10.81
16.00
19.00
20.18
12.01
14
14.01
15
11
12
3
4
5
6
7
8 9
10
11
12
13
16
17
18
Na Mg 3B 4B
5B
6B
7B
8B
IB 2B
Al Si
S CI
Ar
39.95
22.99
24.31
26.98
P
30.97
33
28.09
32.07 35.45
34 35
19
20
21
22
23
24
25
26
27
28
29
30
31
32
36
K
Ca
Sc Ti
V
Cr Mn
Fe Co
Ni
Cu
Zn
Ga
Ge
As Se Br
Kr
39.10
40.08 44.96
47.87
50.94
52.00
54.94
55.85
58.93
58.69
63.55
65.38
69.72
72.63
74.92
78.96
83.80
79.90
53
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
54
Rb Sr Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Xe
Sb
118.71 121.76
85.47 87.62 88.91
91.22
92.91
95.96
(98)
101.07
102.91
106.42
107.87 11241
114.82
Te I
127.60 126.90
84 AS
55
56
57
72
73
74
75
76
77
78
79
80
81
82
43
131.29
86
Rn
Hf
Ta
W
Re
Os
Ir
Pt
Au Hg TI
Pb
Cs Ba La
132.91 137.33 138.91
Bi
174.97
180.95
183.84
186.21
190.23
192.22
195.08
196.97 200.59 204.38
207.2
208.98
Po At
(209)
115 116
(210)
(222)
88
89
104
105
106
107
108 109
110
111 112
113
114
117
118
87
Fr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Nh
FI Mc Lv
Ts
Og
Ra Act
(223) (226) (227) (267) (268) (271) (272)
(277) (276)
(281)
(280)
(285)
(26)
(289)
(293)
(290)
(289) (293)
147 148 149 150
119 120
137
145
146
Do Hr
Wh
Fa
Bd
Mi Wf
Pe
Im
296.2 298.8
341.9
368.1
359.9
67
361.7
68
364.6 366.4
69 70
58
59
60
61
62
64
65
66
Ce Pr
Nd
Pm
Sm Eu
Gd
Tb Dy Ho Er Tm Yb
Lu
140.12 140.91
144.24
(145)
157.25
150.36 151.96
94 95
158.93
97
174.97
103
90
91
93
96
98
162.50 164.93 167.26 168.93 173.05
99 100 101 102
Cf Es Fm Md No
(252)
Np
Pu
Am
Cm
Bk
Th
232.04
Lr
Pa U
231.04 238.03 (237) (244) (243) (247) (247) (247)
(257)
(258) (299)
(262)
Elements and Polyatomic fons NOT Accepted by IUPAC
WI
Wh
Whalium
Wolfite
He
Im
Pe
Horsium
Imaginarium
Bindon
Mi
Microgen
Wolfate
Fa
Ba
Do
Dolphium
Micrite
Micrate
ΤΑ
.
Wolfur
Pepsine
Fantarum
WIO
MiO
Mio.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning