Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.2: Amino Acids And The Henderson–hasselbalch Equation: Isoelectric Points
Problem 4P: Hemoglobin has pI=6.8. Does hemoglobin have a net negative charge or net positive charge at pH=5.3?...
Related questions
Question
What are the hybridization of each structures?
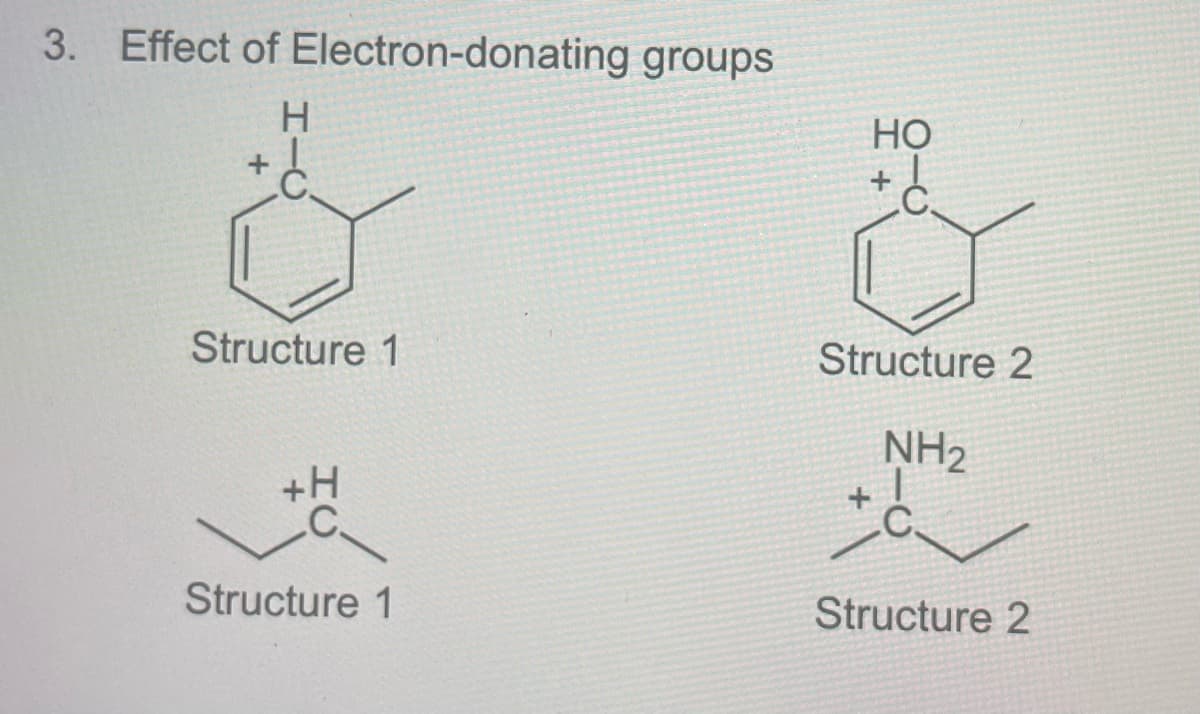
Transcribed Image Text:3. Effect of Electron-donating groups
H.
Но
Structure 1
Structure 2
NH2
+H
+
.C.
Structure 1
Structure 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



