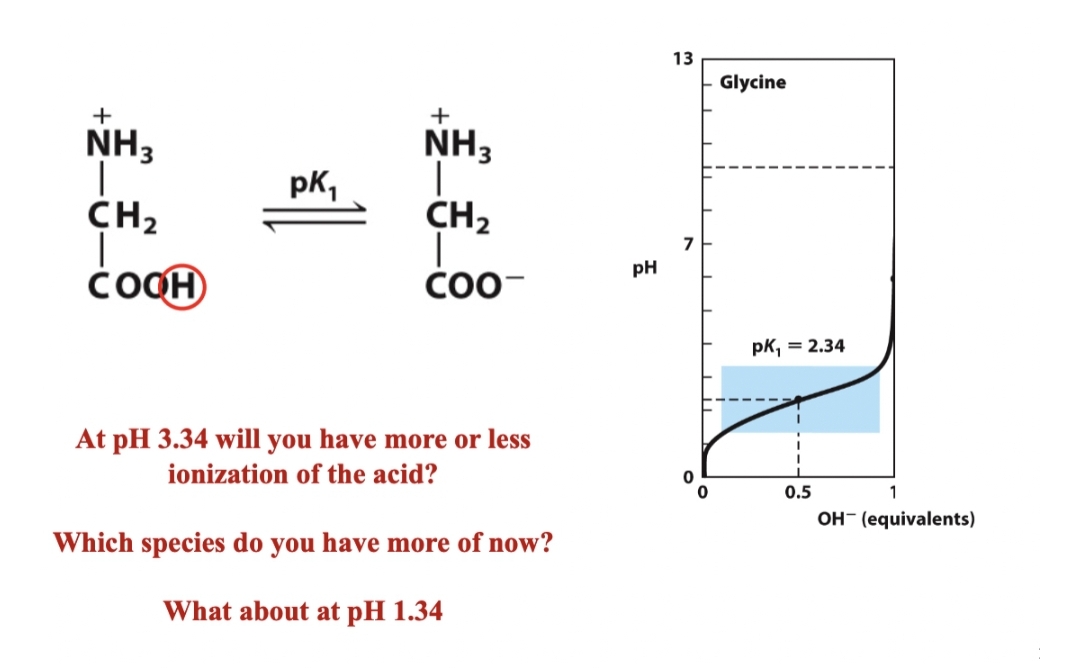
+ + NH3 NH3 pk, CH2 CH2 čoQH ČO0- At pH 3.34 will you have more or less ionization of the acid? Which species do you have more of now? What about at pH 1.34
Q: An acid with a p K a of 8.0 is present in a solution with a pH of 6.0. What is the ratio of the…
A: According to Henderson-Hasselbalch equation pH = pKa + log [A-]/[HA] Given values…
Q: Describe, stepwise, how you would prepare 0.5 L of a 0.15 M phosphate buffer, pH 12.5, using…
A: The equilibrium between the weak acid and its conjugate base allows the solution to resist changes…
Q: When comparing pH, a solution with a pH of 5 contains 100 times the amount of H+ when compared to a…
A: pH of a solution is nothing but the negative log of hydrogen ion concentration in a solution . it…
Q: A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The…
A:
Q: 9. Compare the pH of milk and vinegar, which one is more acidic? How much is the difference between…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: . Compared with a basic solution at pH 9, the same volumeof an acidic solution at pH 4 has _________…
A: pH is a quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The…
Q: If an aqueous solution has a hydroxyl ion concentration of 1 x 10-6 M, what is the concentration of…
A: Water dissociates into hydrogen ions and hydroxyl ions and at neutral pH (pH =7) the numbers of…
Q: What us the formula for pH? note [H+} is hydrogen ion concentration and [K+] is potassium ion…
A: Introduction pH is a measuring scale which is used to determine acidity or basicity of a solution.…
Q: A 0.025 M solution of an unknown organic acid has a pH of 5.70. 1.1 By means of a full calculation,…
A: The Henderson-Hasselbalch equation of a general buffer system; HA↔H+ + A- where 'HA' is the acid and…
Q: Water 1H2O2 is more polar than hydrogen sulfide 1H2S2. Explain.
A: Polarity is the separation of electric charge leading to a molecule or its chemical groups having an…
Q: The concentration of acetic acid (pKa = 4.75) in vinegar is about 1.0 M. What is the pH of vinegar?
A: The pH of a solution is defined as the negative logarithm of the H3O+ ion concentration in moles per…
Q: 3. Normally the pH of the human body is fixed in a very narrow range between 7.35 and 7.45. A…
A: pH stands for potential of hydrogen. It measures the concentration of hydrogen ions present in the…
Q: How much water must be added to 300 mL of an aqueous solution of 0.2 M acetic acid in order to…
A: Given : 0.2 M acetic acid solution , volume 300 ml Let C1 = 0.2 M C2 = concentration of acetic acid…
Q: What's the ratio? An acid with a pKapKa of 8.0 is present in a solution with a pHPH of 6.0. What is…
A: Acids are hydrogen-containing substances that donate a proton (hydrogen ion or H+) to another…
Q: Calculate the [H+] and pKa from the pH of a solution of a weak acid – pH of a 0.02M solution of an…
A: An acid dissociation constant (Ka) is a quantitative measure of the strength of an acid in solution.…
Q: Use the solubility rules to determine if NaCl will react with MgBr KOH will react with NaClI MgS…
A: A reaction is said to occur if there is a formation of an insoluble solid or a precipitate(s) or a…
Q: An aqueous solution contains 0.34 M potassium cyanide. One liter of this solution could be converted…
A: pH is the potential of hydrogen. It is measured by a pH meter. A pH scale ranges from 0 to 14. pH…
Q: Calculate the pH of a buffer that contains 0.15 M MOPS anionic form and 0.25M MOPS zwitterion. The…
A: The zwitterion is the form of amino acid which has net charge of zero. The Henderson-Hasselbalch…
Q: Calculate the concentration of Y4-ion in 0.0100 M EDTA solution at pH 6. α4 = 2.2 x 10-5 for pH 6.
A: Introduction Fraction of EDTA in the form of Y4- is given as αY4-= [Y4-][EDTA]
Q: Make 100 ml of a 1% solution of boric acid isotonic with blood by adding NaCl. The freezing point of…
A: A drug, often known as a medicine, is a chemical compound that has a biological effect on the human…
Q: Would the carbonatelcarbonic acid conjugate base/weak acid system function as a good buffer at the…
A: A buffer is an aqueous solution that is formed of the mixture of weak acid and its conjugate base or…
Q: A very weak acid has à Ka= 4.37 x M? (-) What is the [H+] of a solution of this acid that is 2.65
A: The acids are substances that can readily donate a proton. Bases are molecules that receive a…
Q: Which of the following ionizes completely in solution and is considered to be a strong acid? Select…
A: Acid Acid are those compounds which give hydrogen ion when dissolved in water.
Q: which functional group remains charged at pH of 9. „NH2 H2N COOH
A: The pKa of the ionizable groups on an amino acid determines the net charge of the amino acid. Since…
Q: How do I prepare a 1 M ammonium acetate buffer with a pH of 7. I have solid ammonium acetate, .1 M…
A: Ammonium acetate buffer: Ammonium acetate buffer can be prepared from the reaction of ammonia with…
Q: The spectator ions in the reaction between aqueous perchloric acid and aqueous barium hydroxide are:…
A: 2-
Q: Acetic acid is the principal ingredient in vinegar as shown; that's why it tastes sour. At…
A: An acid dissociation constant (Ka) is the equilibrium constant for a chemical reaction which is…
Q: In mixing compounds with water, the polarity of the compounds helps predict their solubility. When a…
A: Covalent bonds:- When the electron pairs are shared by the atoms, then a covalent bond is formed.…
Q: Are there any H3O+ ions present in pure water at neutral pH (i.e., at pH = 7.0)? If so, how are they…
A: Pure water is also referred as either the distilled water or deionized water. In the distilled…
Q: 1. What is acid indigestion? How is it treated, and explain how the treatment works. 2. What would…
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: A solution with a pH of 2 has a hydrogen ion concentration that is _______________ the hydrogen ion…
A: pH is a measure of the negative logarithm of the hydrogen ion concentration. Given Values: pH = 2.0…
Q: Which of the following aqueous solutions has the lowest pH: 0.1 M HCl; 0.1 M acetic acid (pKa =…
A: pH is used to specify the acidity or basicity of an aqueous solution. It measures the hydrogen ion…
Q: Enter be balanced equation for the ionization of butanoic acid, carboxylic acid, in water.…
A: Carboxylic acid comes under organic acids and contains carboxyl group(C(O=)OH). The general formula…
Q: Write equations showing how each weak base ionizes water to form OH-. Also write the corresponding…
A: A chemical basic substance that does not dissociate completely when dissolved in water is referred…
Q: What is the concentration in % (w/v) of a solution prepared from 50.0 g NaCl and 2.5 L of water? A.…
A: Percentage: The calculation of percentage is one of the simplest and most useful of mathematical…
Q: A solution has a hydrogen ion concentration of 0.01 mol/L. What is its pH? What is its hydroxide ion…
A: Given Values: [H+] = 0.01 Mol/L = 1×10-2 M pH is a measure of negative logarithm of hydrogen ion…
Q: Calculate the pH of a solution if [H3O+] = 3.4 x 10-2M Indicate whether the solution is acidic,…
A: To calculate the pH of the solution The concentration of hydronium ion given as 3.2×10-2M.
Q: 780 mL of a solution that is 0.0160 M in K, starting with solid K4 Fe (CN),- Dissolve g K4 Fe (CN)6…
A: Molarity = n/Volume of solution(in L) n = number of moles 1 L = 1000 ml 1 ml = 0.001 L 780 ml = 0.78…
Q: Which of the following solutions has the greatest concentration of hydroxyl ions [OH-]? Select one:…
A: A solution can be acidic or basic depends upon the concentration of H+ or OH- ions.
Q: When the pH is acidic, the concentration of H+ is high and functional group(s) [ Select ] will be…
A: Amino acids is Organic compound they have both acidic and basic functional groups that is why this…
Q: The amino acid alanine has two isomers, α-alanine and β-alanine. When equal masses of these two…
A: Amino acids are the monomers of proteins having chiral carbon with all four different groups except…
Q: Why doesn't frozen water sink in liquid water? Is the liquid phase of water more or less dense than…
A: Most substances are more dense in their solid form than their liquid forms. This is not seen in the…
Q: What is the pH of a buffer prepared by adding 30.0 mL of 0.25 M acetic acid (CH₂COOH) (K. = 1.7 x…
A: The pH of a buffer prepared by adding 30.0 mL of 0.25 M acetic acid to 125.0 mL of 0.15 M sodium…
Q: Beer’s law is strictly obeyed only in dilute solutions. Why?
A: It is frequently expected that Beer's Law is consistently a direct plot portraying the connection…
Q: :OH KMNO4
A: Potassium permanganate (KMnO4) is a very strong oxidant which is able to react with many functional…
Q: The H⁺ concentration in an aqueous solution at 25 °C is 4.3 × 10⁻⁶. What is [OH⁻]?
A: Hydrogen ion concentration [H+] of a solution is expressed in terms of pH. pH is the negative log of…
Q: The pOH of a solution of NaOH is 11.30. What is the [H+] for this solution? O 2.0 x 10-3 O 2.5 x…
A: pH is the measure of the concentration of hydrogen ions in a given solution. It is measured on a log…
Q: Calculate the [OH] and the pH of a solution with an [H] = 5.6 x 10-10 M at 25 °C. [OH] = pH =…
A: We are authorized to answer one question at a time, since you have not mentioned which question you…
Q: The pKa values of a compound with two ionizable groups are pK1 = 4.10 and pK2 between 7 and 10. A…
A: The Henderson-hasselbalch equation is:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Figure 2.12 A pH scale. Here, red dots signify hydrogen ions (H+) and blue dots signify hydroxyl ions (OH). Also shown are the approximate pH values for some common solutions. This pH scale ranges from 0 (most acidic) to 14 (most basic). A change of one unit on the scale corresponds to a tenfold change in the amount of H+ ions. Photos, JupiterImages Corporation. Figure It Out: What is the approximate pH of cola?What is the pH of a grapefruit that contains 0.007 M citric acid solution (CHgO7)? C6H307(aq) + H₂O(E)= CsH707 (aq) + H₂O*(aq) K₂=7.5 x 104 Round your answer to 2 decimal places.A 0.10 M solution of the deadly poison hydrogen cyanide, HCN, has a pH of 5.2. Calculate the [H3O+] of the solution. Is HCN a strong or a weak acid?
- A solution with a pH of 6 has a ____ difference in H ion concentration than a solution with a pH of 10. If a solution has a concentration of 10^-7 OH ions, how many H ions does it have?The much-abused drug cocaine is an alkaloid. Alkaloids are noted for their bitter taste, an indication of their basic properties. Cocaine, C17H21O4N, is soluble in water to the extent of 0.17g/100mL solution, and a saturated solution has a pH = 10.08. What is the value of Kb for cocaine?Soft drinks usually have a pH of approximately 3.1. What is the [H3O+] concentration in a soft drink?
- At pH ___, the pentapeptide AEHVC would contain two positively charged groups and one negatively charged group. a) 7 b) 3 c) 1 d) 9 e) 5 The answer was b, but I do not know why.The isoelectric point (pI) of glutamic acid is pH 3.08. Draw the structure of the major form of glutamic acid at pH values of: a. 1.00 b. 3.08 c. 11.00.Acetic acid is the principal ingredient in vinegar as shown; that's why it tastes sour. At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and [H3 O+] = [CH3 CO2−] = 0.00118 M. What is the value of Ka for acetic acid?
- The molecular weight of glucose is 180.16 g/mol. Say you want to make 100 ml of a 0.6M solution of glucose. How much glucose and water should you use? Show your calculations for how you would make this solution.An aqueous solution contains 0.448 M dimethylamine ((CH3)2NH).How many mL of 0.240 M hydroiodic acid would have to be added to 150 mL of this solution in order to prepare a buffer with a pH of 10.500? ______________mlNitrogen (N) normally forms three covalent bonds with a valence of five. However, ammonium has four covalent bonds, each to a different hydrogen (H) atom (H has a valence of one). What do you predict to be the charge on ammonium?



