A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.00 mL of a 0.490 M HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is 4.740. Express your answer numerically to two decimal places. Use a minus ( - ) sign if the pH has decreased. • View Available Hint(s) ΑΣφ ApH =
A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.00 mL of a 0.490 M HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is 4.740. Express your answer numerically to two decimal places. Use a minus ( - ) sign if the pH has decreased. • View Available Hint(s) ΑΣφ ApH =
Chapter11: Health Of Aquatic Animals
Section: Chapter Questions
Problem 3KA
Related questions
Question
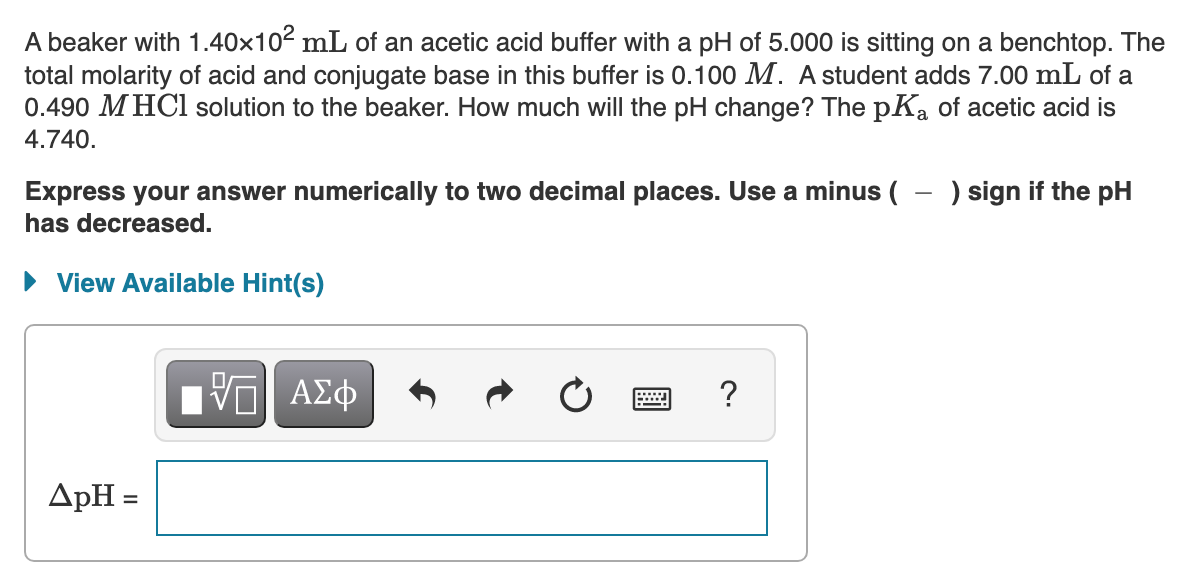
Transcribed Image Text:A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The
total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.00 mL of a
0.490 M HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is
4.740.
Express your answer numerically to two decimal places. Use a minus ( - ) sign if the pH
has decreased.
View Available Hint(s)
ΑΣφ
ApH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you


Human Biology (MindTap Course List)
Biology
ISBN:
9781305112100
Author:
Cecie Starr, Beverly McMillan
Publisher:
Cengage Learning


Human Biology (MindTap Course List)
Biology
ISBN:
9781305112100
Author:
Cecie Starr, Beverly McMillan
Publisher:
Cengage Learning