Nitric acid, HNO3, is produced from ammonia (NH3) and oxygen gas (O2) How many grams of oxygen gas would have been required to produce 1,000 grams of nitric acid, HNO3? Express your answer in THREE SIGNIFICANT FIGURES.
Nitric acid, HNO3, is produced from ammonia (NH3) and oxygen gas (O2) How many grams of oxygen gas would have been required to produce 1,000 grams of nitric acid, HNO3? Express your answer in THREE SIGNIFICANT FIGURES.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter2: Atoms
Section: Chapter Questions
Problem 2.73P: 2-73 (Chemical Connections 2D) Copper is a soft metal. how can it be made harder?
Related questions
Question
Nitric acid, HNO3, is produced from ammonia (NH3) and oxygen gas (O2)
- How many grams of oxygen gas would have been required to produce 1,000 grams of nitric acid, HNO3? Express your answer in THREE SIGNIFICANT FIGURES.
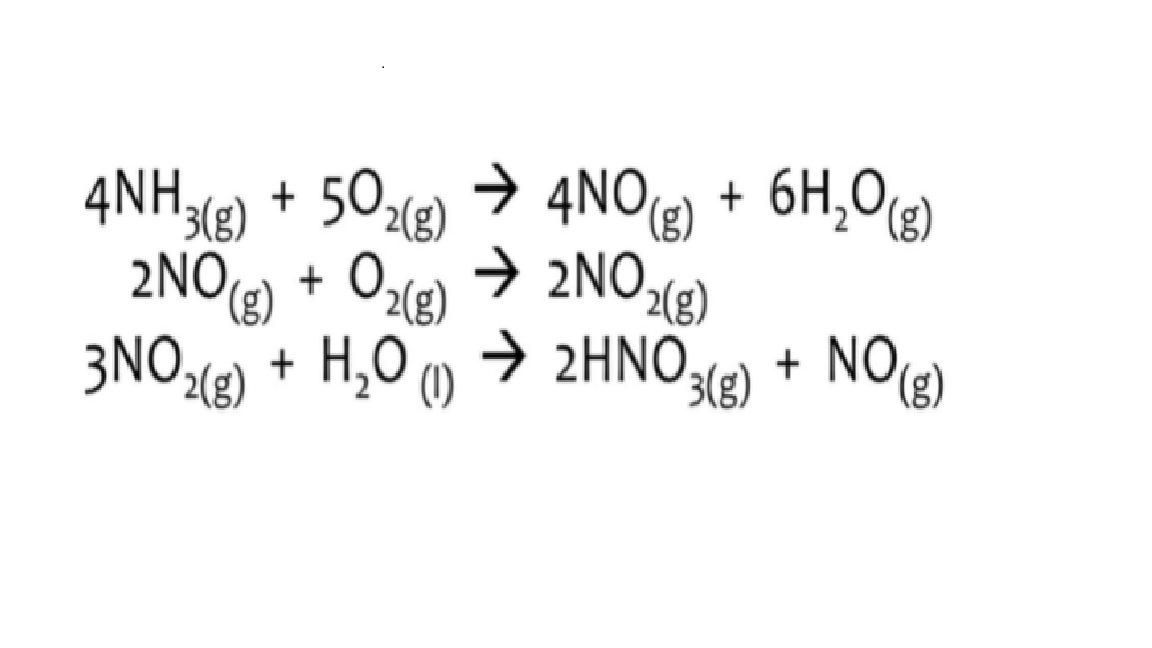
Transcribed Image Text:→ 4NO) + 6H,0g)
4NH3(8)
2NO) + Oz@) → 2NO-(8)
3NO2(e)
2(g)
+ Oz(g)
2(g)
+ NO,
(),
+ H,0 () → 2HNO3(3)
H,O M
3(g)
(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning