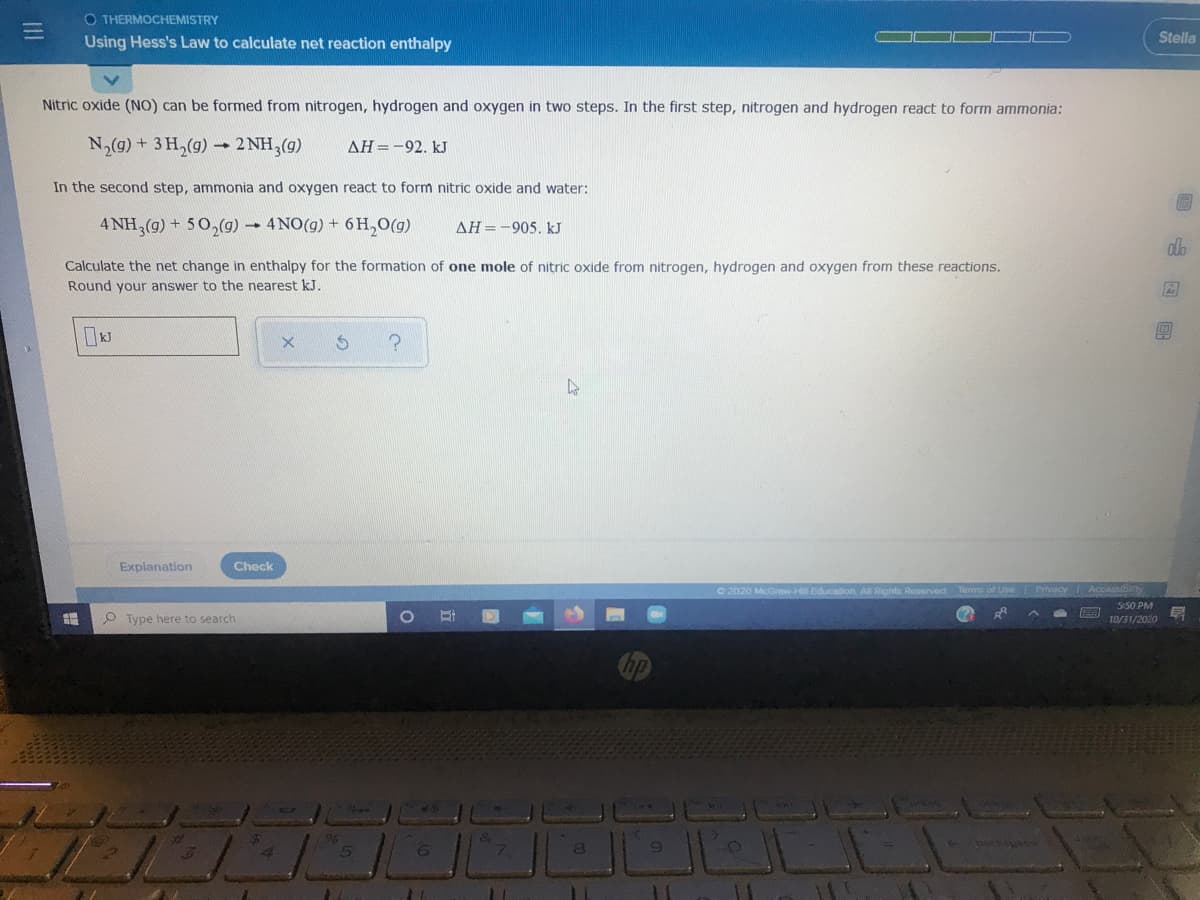
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia: N2(g) + 3 H,(g) - 2 NH,(g) AH=-92. kJ In the second step, ammonia and oxygen react to form nitric oxide and water: 4 NH,(g) + 50,(g) -4NO(g) + 6H,0(g) AH=-905. kJ Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ.
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia: N2(g) + 3 H,(g) - 2 NH,(g) AH=-92. kJ In the second step, ammonia and oxygen react to form nitric oxide and water: 4 NH,(g) + 50,(g) -4NO(g) + 6H,0(g) AH=-905. kJ Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 49QAP: Chlorine trifluoride is a toxic, intensely reactive gas. It was used in World War II to make...
Related questions
Question
100%
Transcribed Image Text:O THERMOCHEMISTRY
Using Hess's Law to calculate net reaction enthalpy
Stella
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia:
N,(g)+ 3H,(g)-
- 2 NH,(g)
AH=-92. kJ
In the second step, ammonia and oxygen react to form nitric oxide and water:
4 NH,(g) + 50,(g) 4NO(g) + 6 H,0(g)
AH=-905. kJ
Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions.
Round your answer to the nearest kJ.
Explanation
Check
O 2020 McGraw-Hill
cation All Rights Reserved
s of Use Privacy
Accassibty
550 PM
P Type here to search
10/31/2020
回 国回
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
