Nó Chloroplasts 0.45 Chloroplasts in the 0.4 dark Chloroplasts in the light 0.35 0.3 10 15 Time (minutes) w would you interpret the above data? Results indicate that the herbicide: a. interferes with the Calvin cycle and prevents electron transport b. interferes with the light reactions and prevents electron transport c had no effect on photosynthesis d. increased the rate of photosynthesis e. may have inhibited photosynthesis but the controls indicate the chloroplasts were not reducing DCPIP therefore the experiment wa herbicide 20
Nó Chloroplasts 0.45 Chloroplasts in the 0.4 dark Chloroplasts in the light 0.35 0.3 10 15 Time (minutes) w would you interpret the above data? Results indicate that the herbicide: a. interferes with the Calvin cycle and prevents electron transport b. interferes with the light reactions and prevents electron transport c had no effect on photosynthesis d. increased the rate of photosynthesis e. may have inhibited photosynthesis but the controls indicate the chloroplasts were not reducing DCPIP therefore the experiment wa herbicide 20
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 18P: Distinguishing the Mechanisms of Class I and Class I Aldolases Fructose bisphosphate aldolase in...
Related questions
Question
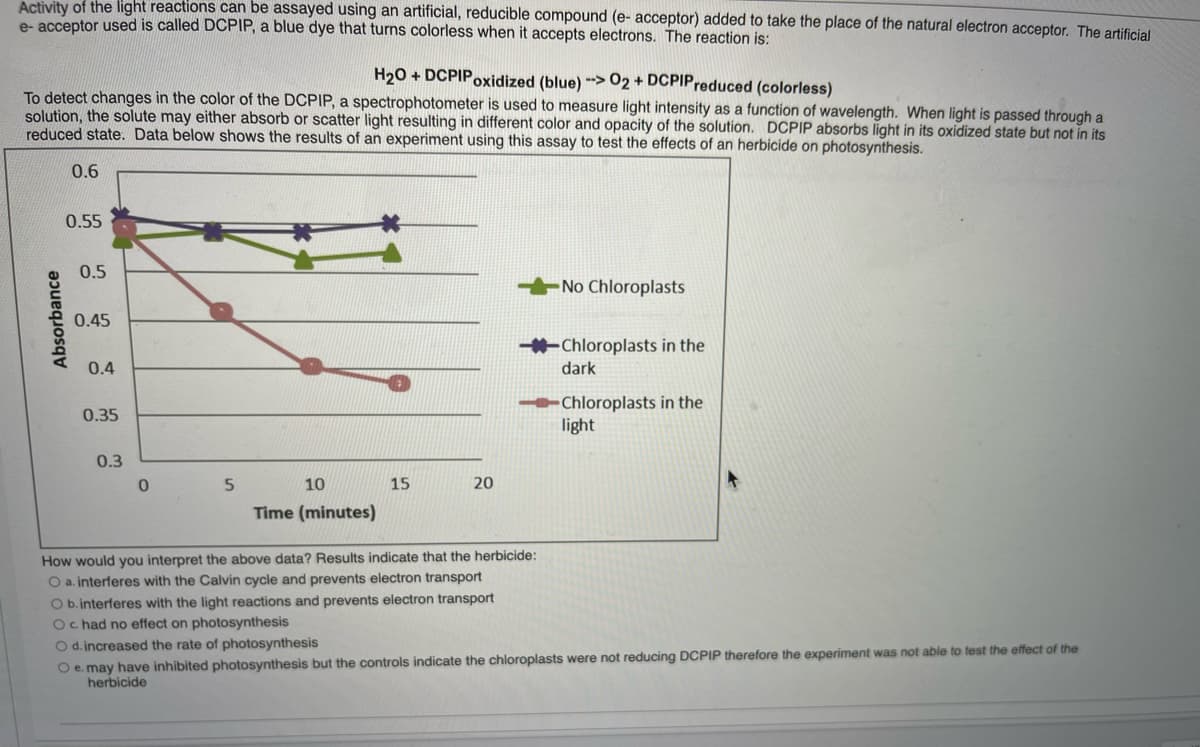
Transcribed Image Text:Activity of the light reactions can be assayed using an artificial, reducible compound (e- acceptor) added to take the place of the natural electron acceptor. The artificial
e- acceptor used is called DCPIP, a blue dye that turns colorless when it accepts electrons. The reaction is:
H20 + DCPIPoxidized (blue) ->02 + DCPIPreduced (colorless)
To detect changes in the color of the DCPIP, a spectrophotometer is used to measure light intensity as a function of wavelength. When light is passed through a
solution, the solute may either absorb or scatter light resulting in different color and opacity of the solution. DCPIP absorbs light in its oxidized state but not in its
reduced state. Data below shows the results of an experiment using this assay to test the effects of an herbicide on photosynthesis.
0.6
0.55
0.5
No Chloroplasts
0.45
Chloroplasts in the
0.4
dark
Chloroplasts in the
light
0.35
0.3
10
15
20
Time (minutes)
How would you interpret the above data? Results indicate that the herbicide:
O a. interferes with the Calvin cycle and prevents electron transport
O b.interferes with the light reactions and prevents electron transport
Oc had no effect on photosynthesis
O d. increased the rate of photosynthesis
O e. may have inhibited photosynthesis but the controls indicate the chloroplasts were not reducing DCPIP therefore the experiment was not able to test the effect of the
herbicide
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning