No. 3. Consider this item as ideal gas: A drum 6 inches in diameter and 40 inches long contained acetylene at 250 psia and 80°F. After some of the acetylene was used the pressure was 200 psia and the temperature was 70°F (a) what portion of the acetylene was used, and (b) what volume would the used acetylene occupy at 14.7 psia and 60°F. R(acetylene) = 59.35 ft-lbf/lbm-R Answer: a. b. V = % mass
No. 3. Consider this item as ideal gas: A drum 6 inches in diameter and 40 inches long contained acetylene at 250 psia and 80°F. After some of the acetylene was used the pressure was 200 psia and the temperature was 70°F (a) what portion of the acetylene was used, and (b) what volume would the used acetylene occupy at 14.7 psia and 60°F. R(acetylene) = 59.35 ft-lbf/lbm-R Answer: a. b. V = % mass
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter9: Refrigerant And Oil Chemistry And Management-recovery, Recycling,
Section: Chapter Questions
Problem 17RQ: What is the name of the conference that was held in Canada in 1987 to attempt to solve the problem...
Related questions
Question
100%
UPVOTE WILL BE GIVEN. PLEASE WRITE THE COMPLETE SOLUTIONS LEGIBLY. SHOW THE STEP-BY-STEP PROCESS WITH SHORT COMMENT/EXPLANATION. ANSWER IN 3 DECIMAL PLACES.
ANSWER #3 ONLY not all. Thank you.
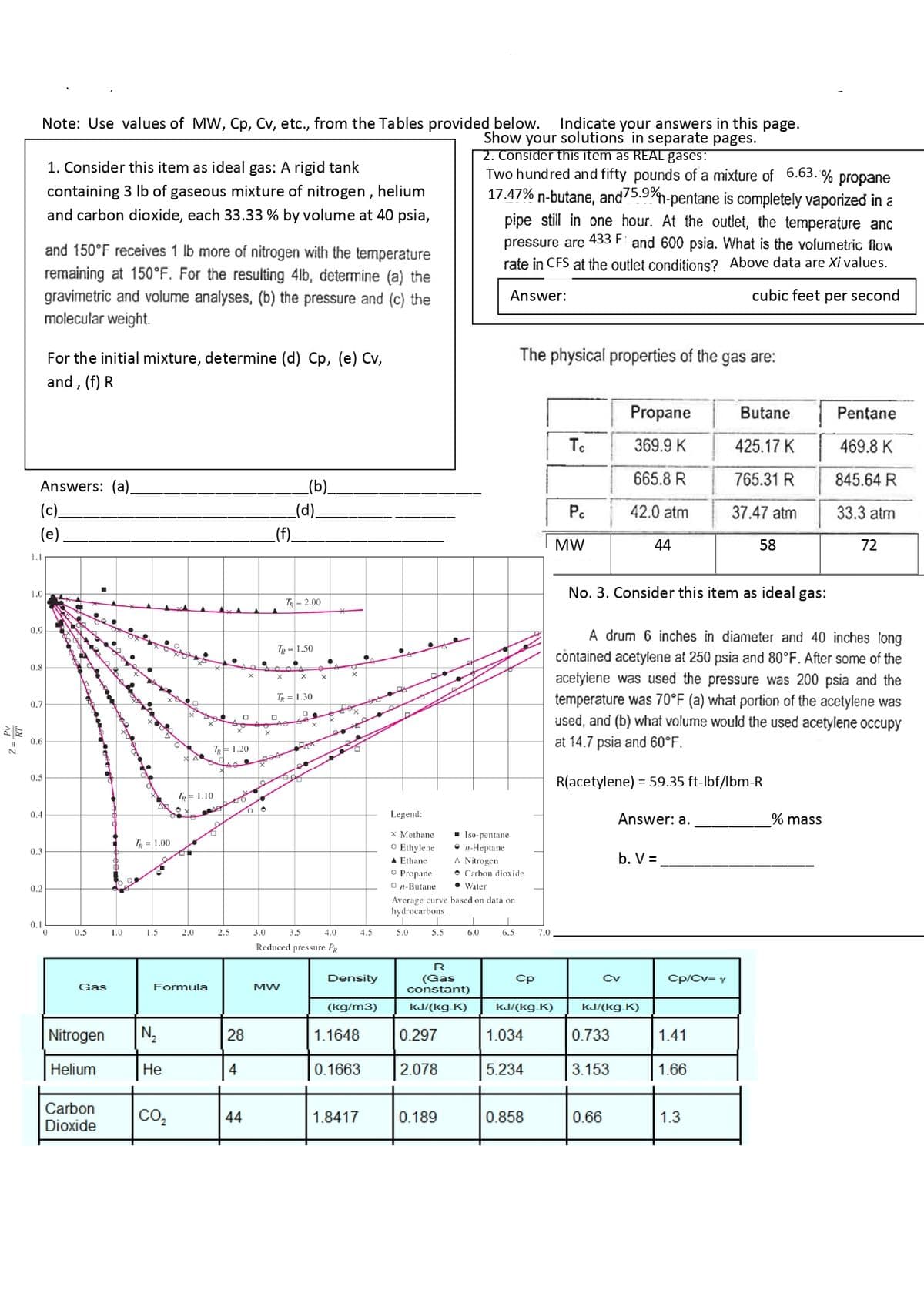
Transcribed Image Text:Z=RT
Note: Use values of MW, Cp, Cv, etc., from the Tables provided below. Indicate your answers in this page.
Show your solutions in separate pages.
2. Consider this item as REAL gases:
Two hundred and fifty pounds of a mixture of 6.63.% propane
17.47% n-butane, and 75.9%-
h-pentane is completely vaporized in a
pipe still in one hour. At the outlet, the temperature anc
pressure are 433 F and 600 psia. What is the volumetric flow
rate in CFS at the outlet conditions? Above data are Xi values.
Answer:
cubic feet per second
1.1
1.0
Answers: (a).
(c).
(e)
0.9
0.8
0.7
0.6
0.5
0.4
0.3
1. Consider this item as ideal gas: A rigid tank
containing 3 lb of gaseous mixture of nitrogen, helium
and carbon dioxide, each 33.33% by volume at 40 psia,
0.2
and 150°F receives 1 lb more of nitrogen with the temperature
remaining at 150°F. For the resulting 4lb, determine (a) the
gravimetric and volume analyses, (b) the pressure and (c) the
molecular weight.
For the initial mixture, determine (d) Cp, (e) Cv,
and, (f) R
0.1
0
0.5
Gas
1.0
TR=1.00
1.5
Nitrogen N₂
Helium
He
Carbon CO₂
Dioxide
TR= 1.10
2.0
Formula
L
TR=1.20
aque
y
X
2.5
oot
28
4
44
C
X
Do
3.0
(f).
X
_(b)_
_(d)_
TR= 1.50
●
TR= 2.00
MW
TR=1.30
3.5 4.0
Reduced pressure PR
DAX
4.5
Density
(kg/m3)
1.1648
0.1663
1.8417
Legend:
x Methane
o Ethylene
▲ Ethane
O Propane
On-Butane
5.0
• Water
Average curve based on data on
hydrocarbons
5.5
■ Iso-pentane
• n-Heptane
A Nitrogen
0.297
2.078
0.189
Carbon dioxide.
R
(Gas
constant)
kJ/(kg.K)
6.0
The physical properties of the gas are:
I
6.5
Cp
kJ/(kg.K)
1.034
5.234
7.0
0.858
Tc
Pc
MW
Propane
369.9 K
665.8 R
42.0 atm
No. 3. Consider this item as ideal gas:
0.733
3.153
0.66
44
Cv
A drum 6 inches in diameter and 40 inches long
contained acetylene at 250 psia and 80°F. After some of the
acetylene was used the pressure was 200 psia and the
temperature was 70°F (a) what portion of the acetylene was
used, and (b) what volume would the used acetylene occupy
at 14.7 psia and 60°F.
R(acetylene) = 59.35 ft-lbf/lbm-R
Answer: a.
kJ/(kg.K)
b. V =
Cp/Cv=Y
1.41
Butane
425.17 K
765.31 R
37.47 atm
58
1.66
1.3
Pentane
469.8 K
845.64 R
33.3 atm
% mass
72
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning