-
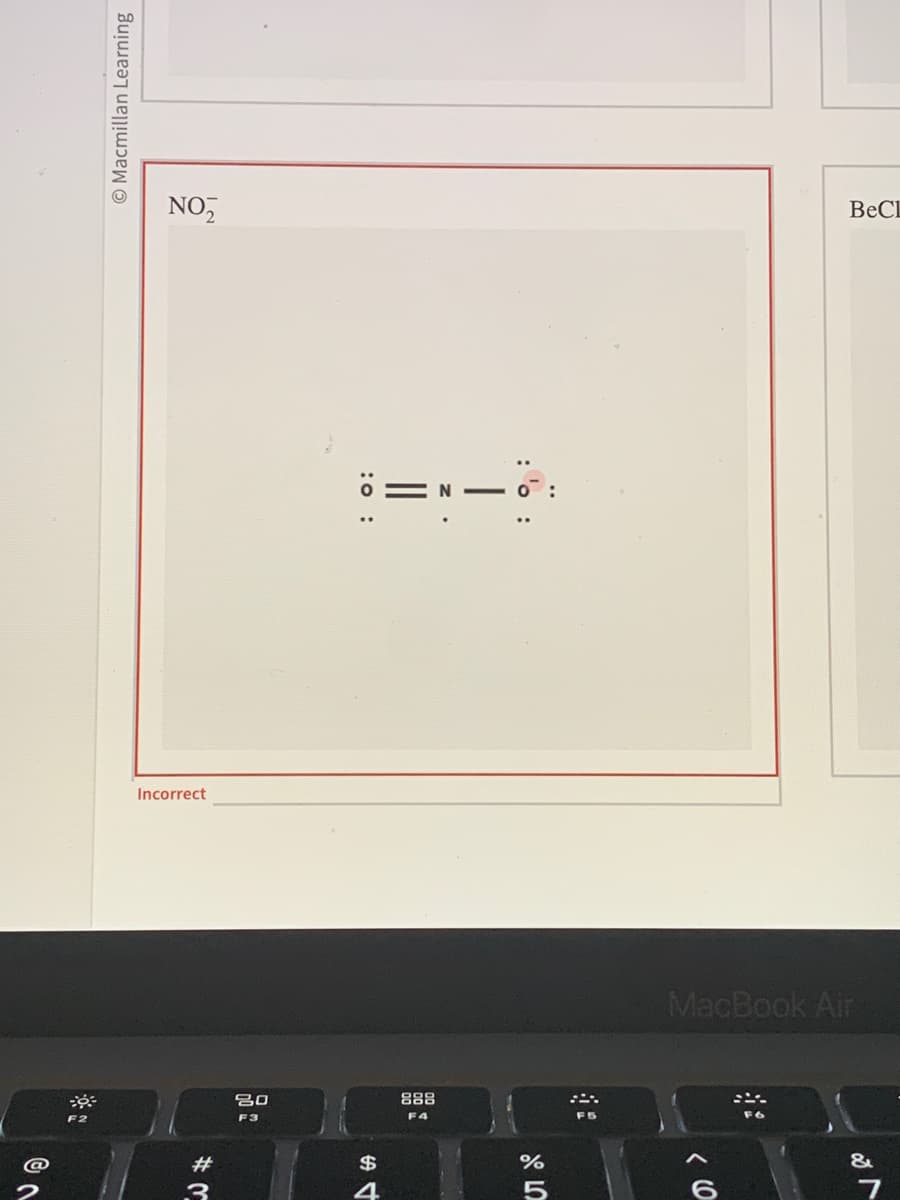
Determine the number of valence electrons: The number of valence electrons can be determined from the periodic table, and is the number of electrons in the outermost shell of an atom.
-
Determine the skeletal structure: Determine the skeletal structure of the molecule, placing the atoms in the appropriate order to satisfy the octet rule (the idea that atoms tend to bond in such a way that each atom has eight electrons in its outermost shell).
-
Place bonding pairs: For each bond between two atoms, place a pair of electrons between them to form a bond.
-
Place non-bonding electrons: For each atom, add any remaining electrons as non-bonding electrons (also known as lone pairs).
-
Assign formal charges: Assign formal charges to each atom in the structure, based on the number of electrons it has relative to the number of electrons it would have in a neutral atom of the same element.
-
Optimize the structure: Optimize the Lewis structure by rearranging electrons and double bonds in such a way as to minimize the formal charges on the atoms.
Step by step
Solved in 2 steps with 1 images









