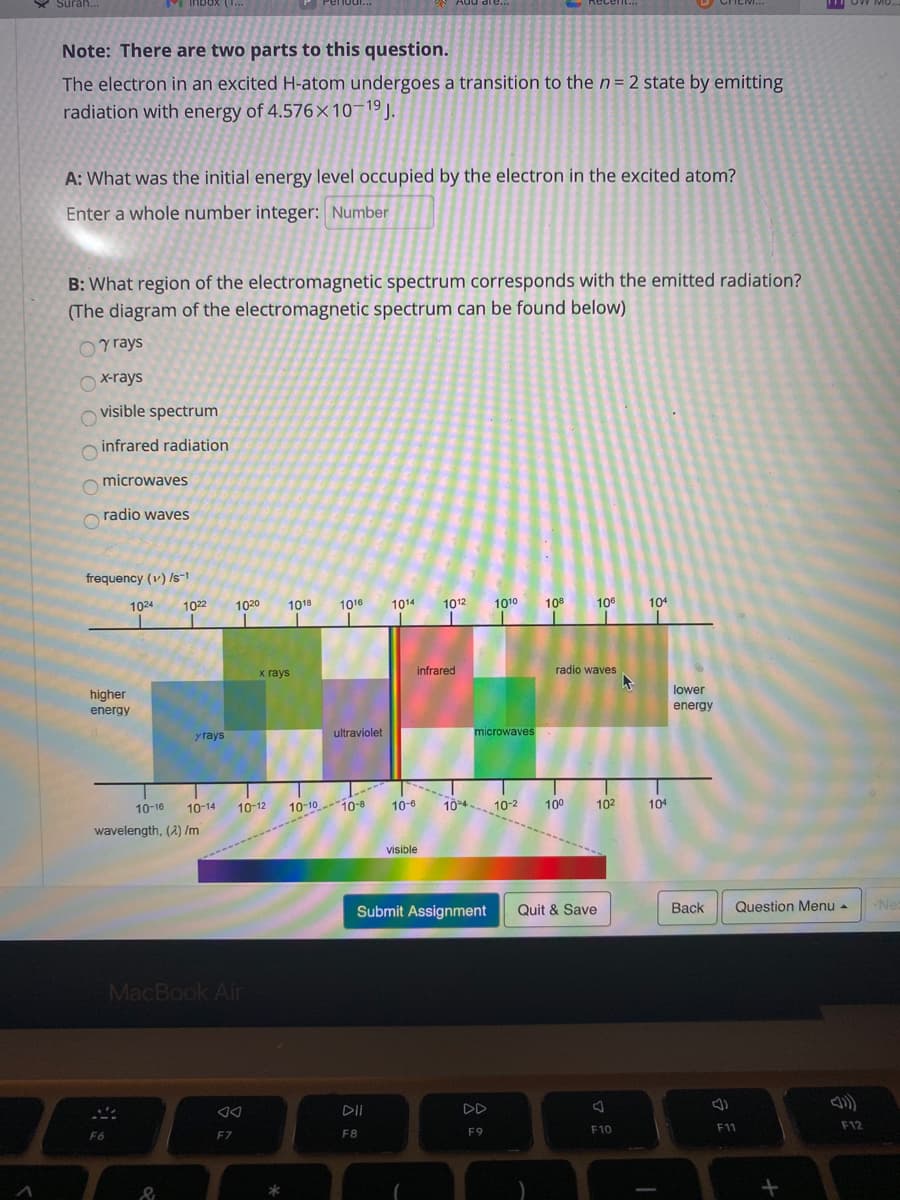
Note: There are two parts to this question. The electron in an excited H-atom undergoes a transition to the n= 2 state by emitting radiation with energy of 4.576×10¬19J. A: What was the initial energy level occupied by the electron in the excited atom? Enter a whole number integer: Number B: What region of the electromagnetic spectrum corresponds with the emitted radiation? (The diagram of the electromagnetic spectrum can be found below) Oy rays x-rays visible spectrum infrared radiation microwaves radio waves
Note: There are two parts to this question. The electron in an excited H-atom undergoes a transition to the n= 2 state by emitting radiation with energy of 4.576×10¬19J. A: What was the initial energy level occupied by the electron in the excited atom? Enter a whole number integer: Number B: What region of the electromagnetic spectrum corresponds with the emitted radiation? (The diagram of the electromagnetic spectrum can be found below) Oy rays x-rays visible spectrum infrared radiation microwaves radio waves
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.118QP: Warm objects emit electromagnetic radiation in the infrared region. Heat lamps employ this principle...
Related questions
Question
Transcribed Image Text:Surah.
M Inbox
Note: There are two parts to this question.
The electron in an excited H-atom undergoes a transition to the n = 2 state by emitting
radiation with energy of 4.576×10¬19J.
A: What was the initial energy level occupied by the electron in the excited atom?
Enter a whole number integer: Number
B: What region of the electromagnetic spectrum corresponds with the emitted radiation?
(The diagram of the electromagnetic spectrum can be found below)
Oy rays
Oxrays
O visible spectrum
infrared radiation
microwaves
radio waves
frequency (v) /s-1
1024
1022
1020
1018
1016
1014
1012
1010
108
10
104
х гаys
infrared
radio waves
lower
higher
energy
energy
y rays
ultraviolet
microwaves
10-16
10-14
10-12
10-1010-8
10-6
104 10-2
100
102
104
wavelength, (2) /m
visible
Submit Assignment
Quit & Save
Back
Question Menu -
Ne
MacBook Air
DII
DD
F10
F11
F12
F6
F7
F8
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
