Which of the following reactions is NOT correct?
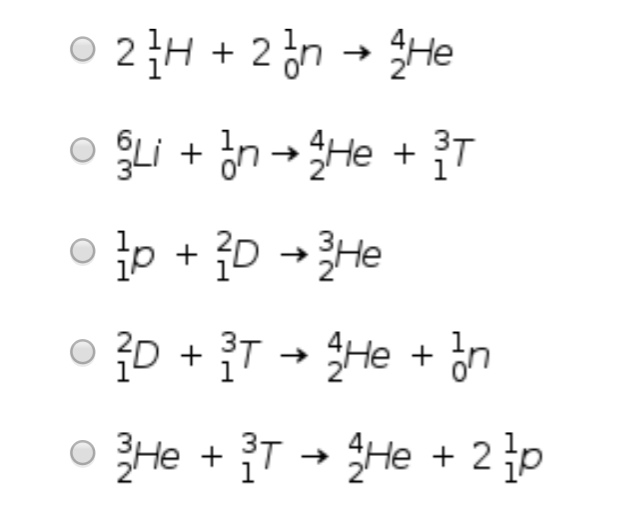
Since in any reaction,
1) total mass number of the reactants = total mass number of the products
2) total Atomic number of reactants = total atomic number of products
This is because both atomic and mass number represents the subatomic particle and in any reaction, subatomic particles neither can be created nor destroyed
Mass number is represented by the top left number and atomic number by bottom left number written around the species
A) 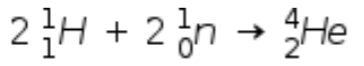
Here we have total mass number of product = 4
And mass number of reactant = 2 X 1 + 2 X 1 = 4 ( because we have 2 H and 2 n so multiplying by 2 with mass numbers)
And total atomic number of product = 2
And atomic number of reactant = 2 X 1 + 2 X 0 = 2
hence the condition is satisfied. => this is correct reaction
B) 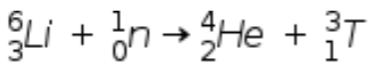
Here we have total mass number of product = 4 + 3 = 7
And mass number of reactant = 6 + 1 = 7
And total atomic number of product = 3 + 0 = 3
And atomic number of reactant = 2+1 = 3
hence the condition is satisfied. => this is correct reaction
Step by step
Solved in 6 steps with 5 images







