O Attempt 2 Some of the formulas could be either molecular or empirical formulas; however, some can only be molecular formulas. Which of the formulas must be molecular formulas? O H,O2 V C,H1206 РО C,H,N,0 C,H, Incorrect
O Attempt 2 Some of the formulas could be either molecular or empirical formulas; however, some can only be molecular formulas. Which of the formulas must be molecular formulas? O H,O2 V C,H1206 РО C,H,N,0 C,H, Incorrect
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 21ALQ: True or false? The atom with the largest subscript in a formula is the atom with the largest percent...
Related questions
Question

Transcribed Image Text:O Attempt 2
Some of the formulas could be either molecular or empirical formulas; however, some can only be molecular formulas.
Which of the formulas must be molecular formulas?
O H,02
V C,H1206
PO
C,H,N,0
C,H,
Incorrect
Expert Solution
Step 1
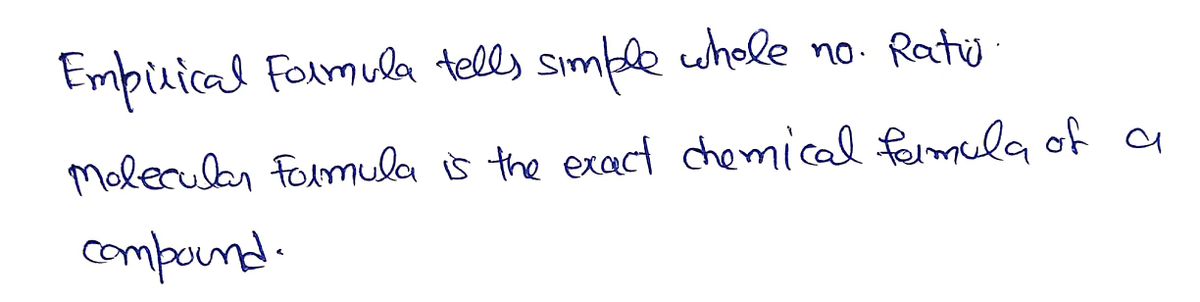
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning