Chapter25: Biomolecules: Carbohydrates
Section25.SE: Something Extra
Problem 69AP
Related questions
Question
Draw the curly arrow mechanism for this experiment, include all details like electrons, curly arrows, by products etc
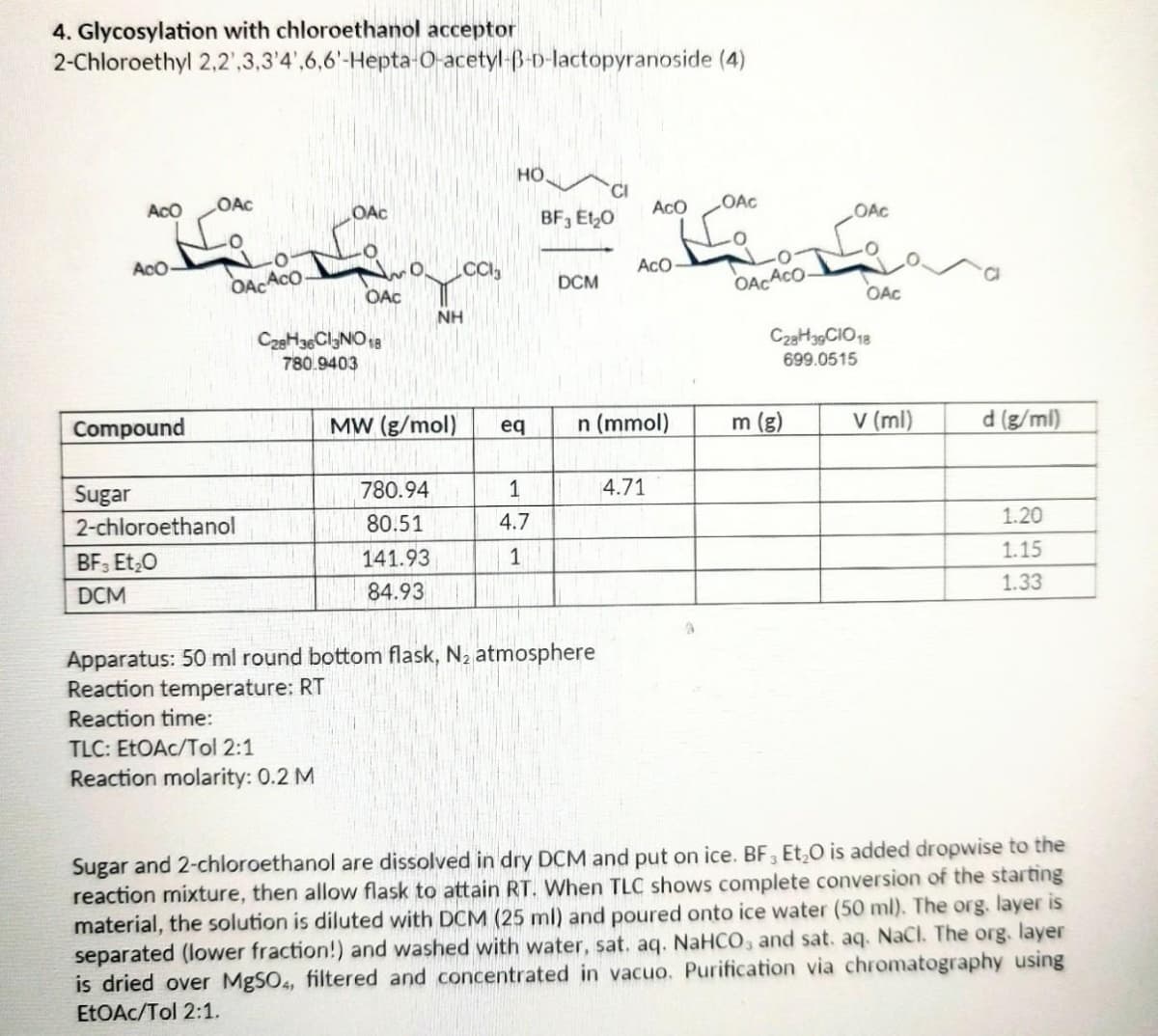
Transcribed Image Text:4. Glycosylation with chloroethanol acceptor
2-Chloroethyl
ACO
مایا
2,2,3,3'4',6,6'-Hepta-O-acetyl-B-D-lactopyranoside (4)
HO
CI
ACO
OAC
AcO-
OAC
OAC
OACACO
OAC
CC13
ACO
NH
Compound
MW (g/mol)
eq
n (mmol)
d (g/ml)
780.94
1
4.71
Sugar
2-chloroethanol
80.51
4.7
1.20
BF3 Et₂O
141.93
1
1.15
1.33
DCM
84.93
Apparatus: 50 ml round bottom flask, N₂ atmosphere
Reaction temperature: RT
Reaction time:
TLC: EtOAc/Tol 2:1
Reaction molarity: 0.2 M
Sugar and 2-chloroethanol are dissolved in dry DCM and put on ice. BF3 Et₂O is added dropwise to the
reaction mixture, then allow flask to attain RT. When TLC shows complete conversion of the starting
material, the solution is diluted with DCM (25 ml) and poured onto ice water (50 ml). The org. layer is
separated (lower fraction!) and washed with water, sat. aq. NaHCO3 and sat. aq. NaCl. The org. layer
is dried over MgSO4, filtered and concentrated in vacuo. Purification via chromatography using
EtOAc/Tol 2:1.
C28H36Cl3NO 18
780.9403
BF3 E1₂0
DCM
OACACO-
OAC
m (g)
OAC
C28H39 CIO 18
699.0515
v (ml)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning