Object A specific heat is 2.11J and the object B specific heat is 5.60J Which object will heat up faster if they have the same mass and equal amount of heat is applied and why?
Object A specific heat is 2.11J and the object B specific heat is 5.60J Which object will heat up faster if they have the same mass and equal amount of heat is applied and why?
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter14: Heat And Heat Transfer Methods
Section: Chapter Questions
Problem 53PE: A person inhales and exhales 2.00 L of 37.0C air, evaporating 4.00102g of water from the lungs and...
Related questions
Question
Object A specific heat is 2.11J and the object B specific heat is 5.60J Which object will heat up faster if they have the same mass and equal amount of heat is applied and why?
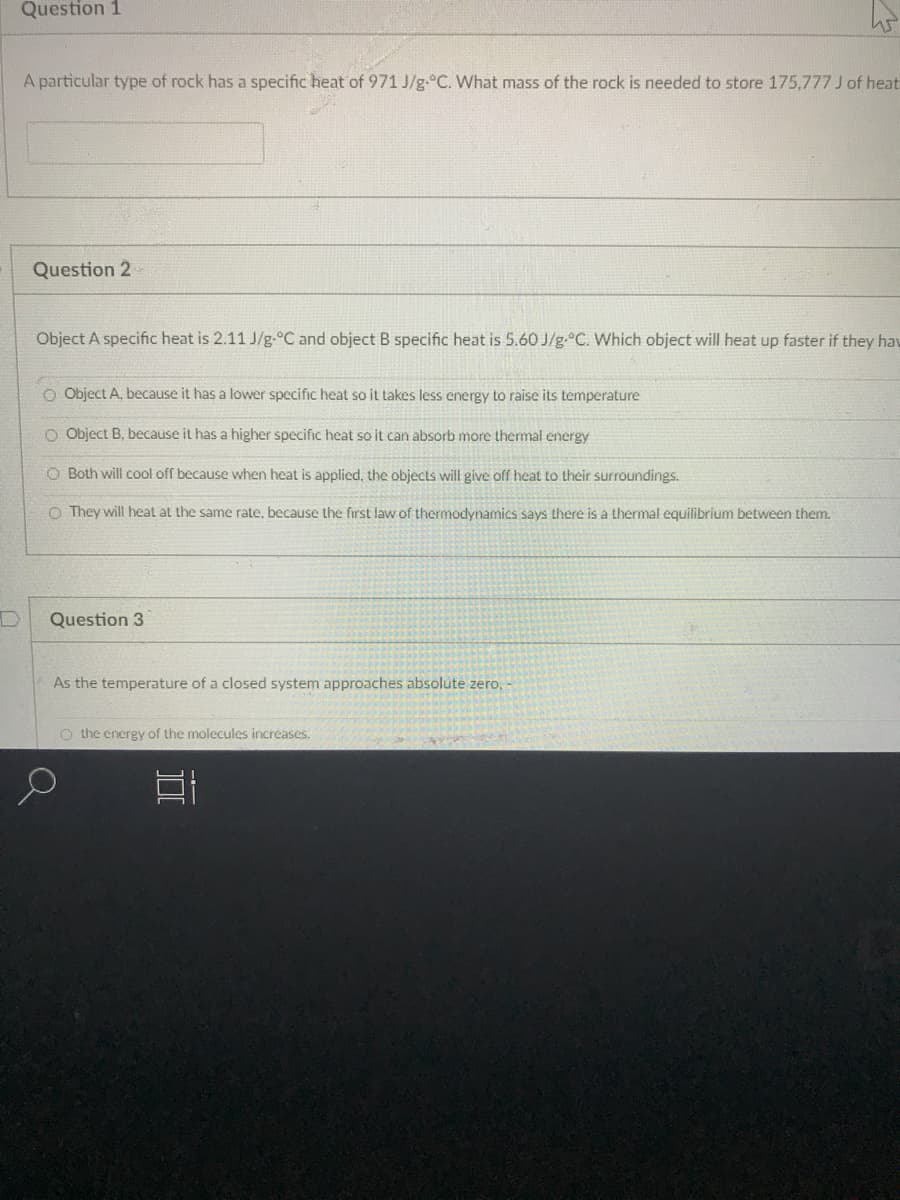
Transcribed Image Text:Question 1
A particular type of rock has a specific heat of 971 J/g.°C. What mass of the rock is needed to store 175,777 J of heat
Question 2
Object A specific heat is 2.11 J/g-°C and object B specific heat is 5.60 J/g.°C. Which object will heat up faster if they hav
O Object A, because it has a lower specific heat so it takes less energy to raise its temperature
O Object B, because it has a higher specific heat so it can absorb more thermal energy
O Both will cool off because when heat is applied, the objects will give off heat to their surroundings.
O They will heat at the same rate, because the first law of thermodynamics says there is a thermal equilibrium between them.
Question 3
As the temperature of a closed system approaches absolute zero,
O the energy of the molecules increases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning